Plasma metabolomics supports the use of long-duration cardiac arrest rodent model to study human disease by demonstrating similar metabolic alterations
- PMID: 33184308
- PMCID: PMC7665036
- DOI: 10.1038/s41598-020-76401-x
Plasma metabolomics supports the use of long-duration cardiac arrest rodent model to study human disease by demonstrating similar metabolic alterations
Abstract
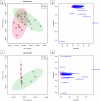
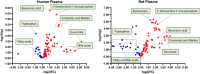
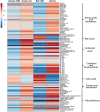
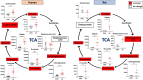
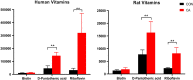
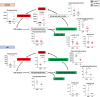
Cardiac arrest (CA) is a leading cause of death and there is a necessity for animal models that accurately represent human injury severity. We evaluated a rat model of severe CA injury by comparing plasma metabolic alterations to human patients. Plasma was obtained from adult human control and CA patients post-resuscitation, and from male Sprague-Dawley rats at baseline and after 20 min CA followed by 30 min cardiopulmonary bypass resuscitation. An untargeted metabolomics evaluation using UPLC-QTOF-MS/MS was performed for plasma metabolome comparison. Here we show the metabolic commonality between humans and our severe injury rat model, highlighting significant metabolic dysfunction as seen by similar alterations in (1) TCA cycle metabolites, (2) tryptophan and kynurenic acid metabolites, and (3) acylcarnitine, fatty acid, and phospholipid metabolites. With substantial interspecies metabolic similarity in post-resuscitation plasma, our long duration CA rat model metabolically replicates human disease and is a suitable model for translational CA research.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Tissue-Specific Metabolic Profiles After Prolonged Cardiac Arrest Reveal Brain Metabolome Dysfunction Predominantly After Resuscitation.J Am Heart Assoc. 2019 Sep 3;8(17):e012809. doi: 10.1161/JAHA.119.012809. Epub 2019 Aug 31. J Am Heart Assoc. 2019. PMID: 31475603 Free PMC article.
-
Examination of physiological function and biochemical disorders in a rat model of prolonged asphyxia-induced cardiac arrest followed by cardio pulmonary bypass resuscitation.PLoS One. 2014 Nov 10;9(11):e112012. doi: 10.1371/journal.pone.0112012. eCollection 2014. PLoS One. 2014. PMID: 25383962 Free PMC article.
-
Phospholipid alterations in the brain and heart in a rat model of asphyxia-induced cardiac arrest and cardiopulmonary bypass resuscitation.Mol Cell Biochem. 2015 Oct;408(1-2):273-81. doi: 10.1007/s11010-015-2505-0. Epub 2015 Jul 10. Mol Cell Biochem. 2015. PMID: 26160279 Free PMC article.
-
1H NMR-metabolomics: can they be a useful tool in our understanding of cardiac arrest?Resuscitation. 2014 May;85(5):595-601. doi: 10.1016/j.resuscitation.2014.01.025. Epub 2014 Feb 7. Resuscitation. 2014. PMID: 24513156 Review.
-
Cardiac arrest: resuscitation and reperfusion.Circ Res. 2015 Jun 5;116(12):2041-9. doi: 10.1161/CIRCRESAHA.116.304495. Circ Res. 2015. PMID: 26044255 Free PMC article. Review.
Cited by
-
A GC-MS-based untargeted metabolomics approach for comprehensive metabolic profiling of vancomycin-induced toxicity in mice.Heliyon. 2022 Jul 6;8(7):e09869. doi: 10.1016/j.heliyon.2022.e09869. eCollection 2022 Jul. Heliyon. 2022. PMID: 35855991 Free PMC article.
-
Pharmacological Approach for Neuroprotection After Cardiac Arrest-A Narrative Review of Current Therapies and Future Neuroprotective Cocktail.Front Med (Lausanne). 2021 May 18;8:636651. doi: 10.3389/fmed.2021.636651. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34084772 Free PMC article. Review.
-
Multi-Drug Cocktail Therapy Improves Survival and Neurological Function after Asphyxial Cardiac Arrest in Rodents.Cells. 2023 Jun 5;12(11):1548. doi: 10.3390/cells12111548. Cells. 2023. PMID: 37296668 Free PMC article.
-
Multi-omics Study of Hypoxic-Ischemic Brain Injury After Cardiopulmonary Resuscitation in Swine.Neurocrit Care. 2025 Feb;42(1):59-76. doi: 10.1007/s12028-024-02038-7. Epub 2024 Jun 27. Neurocrit Care. 2025. PMID: 38937417
-
Serum Metabolomics Reveals Distinct Profiles during Ischemia and Reperfusion in a Porcine Model of Myocardial Ischemia-Reperfusion.Int J Mol Sci. 2022 Jun 16;23(12):6711. doi: 10.3390/ijms23126711. Int J Mol Sci. 2022. PMID: 35743153 Free PMC article.
References
-
- Jacobs I, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa) Resuscitation. 2004;63:233–249. doi: 10.1016/j.resuscitation.2004.09.008. - DOI - PubMed
-
- Patel, K. & Hipskind, J. E. in StatPearls (2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

