Alterations in detrusor contractility in rat model of bladder cancer
- PMID: 33184390
- PMCID: PMC7665011
- DOI: 10.1038/s41598-020-76653-7
Alterations in detrusor contractility in rat model of bladder cancer
Abstract
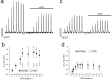
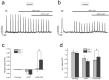
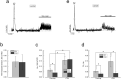
Urinary incontinence of idiopathic nature is a common complication of bladder cancer, yet, the mechanisms underlying changes in bladder contractility associated with cancer are not known. Here by using tensiometry on detrusor smooth muscle (DSM) strips from normal rats and rats with bladder cancer induced by known urothelial carcinogen, N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN), we show that bladder cancer is associated with considerable changes in DSM contractility. These changes include: (1) decrease in the amplitude and frequency of spontaneous contractions, consistent with the decline of luminal pressures during filling, and detrusor underactivity; (2) diminution of parasympathetic DSM stimulation mainly at the expense of m-cholinergic excitatory transmission, suggestive of difficulty in bladder emptying and weakening of urine stream; (3) strengthening of TRPV1-dependent afferent limb of micturition reflex and TRPV1-mediated local contractility, promoting urge incontinence; (4) attenuation of stretch-dependent, TRPV4-mediated spontaneous contractility leading to overflow incontinence. These changes are consistent with the symptomatic of bladder dysfunction in bladder cancer patients. Considering that BBN-induced urothelial lesions in rodents largely resemble human urothelial lesions at least in their morphology, our studies establish for the first time underlying reasons for bladder dysfunction in bladder cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- American Cancer Society https://www.cancer.org/cancer/bladder-cancer/detection-diagnosis-staging...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

