The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19)
- PMID: 33184506
- PMCID: PMC7665044
- DOI: 10.1038/s41598-020-76781-0
The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19)
Abstract
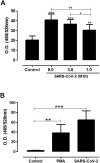
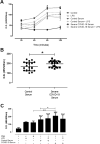
The novel coronavirus SARS-CoV-2 causes COVID-19, a highly pathogenic viral infection threatening millions. The majority of the individuals infected are asymptomatic or mildly symptomatic showing typical clinical signs of common cold. However, approximately 20% of the patients can progress to acute respiratory distress syndrome (ARDS), evolving to death in about 5% of cases. Recently, angiotensin-converting enzyme 2 (ACE2) has been shown to be a functional receptor for virus entry into host target cells. The upregulation of ACE2 in patients with comorbidities may represent a propensity for increased viral load and spreading of infection to extrapulmonary tissues. This systemic infection is associated with higher neutrophil to lymphocyte ratio in infected tissues and high levels of pro-inflammatory cytokines leading to an extensive microthrombus formation with multiorgan failure. Herein we investigated whether SARS-CoV-2 can stimulate extracellular neutrophils traps (NETs) in a process called NETosis. We demonstrated for the first time that SARS-CoV-2 in fact is able to activate NETosis in human neutrophils. Our findings indicated that this process is associated with increased levels of intracellular Reactive Oxygen Species (ROS) in neutrophils. The ROS-NET pathway plays a role in thrombosis formation and our study suggest the importance of this target for therapy approaches against disease.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

