Phenotypic variation of transcriptomic cell types in mouse motor cortex
- PMID: 33184512
- PMCID: PMC8113357
- DOI: 10.1038/s41586-020-2907-3
Phenotypic variation of transcriptomic cell types in mouse motor cortex
Abstract
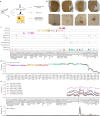
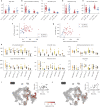
Cortical neurons exhibit extreme diversity in gene expression as well as in morphological and electrophysiological properties1,2. Most existing neural taxonomies are based on either transcriptomic3,4 or morpho-electric5,6 criteria, as it has been technically challenging to study both aspects of neuronal diversity in the same set of cells7. Here we used Patch-seq8 to combine patch-clamp recording, biocytin staining, and single-cell RNA sequencing of more than 1,300 neurons in adult mouse primary motor cortex, providing a morpho-electric annotation of almost all transcriptomically defined neural cell types. We found that, although broad families of transcriptomic types (those expressing Vip, Pvalb, Sst and so on) had distinct and essentially non-overlapping morpho-electric phenotypes, individual transcriptomic types within the same family were not well separated in the morpho-electric space. Instead, there was a continuum of variability in morphology and electrophysiology, with neighbouring transcriptomic cell types showing similar morpho-electric features, often without clear boundaries between them. Our results suggest that neuronal types in the neocortex do not always form discrete entities. Instead, neurons form a hierarchy that consists of distinct non-overlapping branches at the level of families, but can form continuous and correlated transcriptomic and morpho-electrical landscapes within families.
© 2020. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures












References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

