Cholinergic Activation of Primary Human Derived Intestinal Epithelium Does Not Ameliorate TNF-α Induced Injury
- PMID: 33184579
- PMCID: PMC7596162
- DOI: 10.1007/s12195-020-00633-0
Cholinergic Activation of Primary Human Derived Intestinal Epithelium Does Not Ameliorate TNF-α Induced Injury
Abstract
Introduction: The intestinal epithelium contains specialized cells including enterocytes, goblet, Paneth, enteroendocrine, and stem cells. Impaired barrier integrity in Inflammatory Bowel Disease is characterized by elevated levels of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α). Prior studies in immortalized lines such as Caco-2, without native epithelial heterogeneity, demonstrate the amelioration of TNF-α compromised barrier integrity via nicotinic (nAChR) or muscarinic (mAChR) acetylcholine receptor activation.
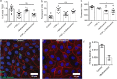
Methods: A tissue-engineered model of primary human small intestinal epithelium was derived from dissociated organoids cultured on collagen-coated Transwells. Differentiation was accomplished with serum-containing media and compared to Caco-2 and HT-29 regarding alkaline phosphatase expression, transepithelial electrical resistance (TEER), and IL-8 secretion. Inflammation was modeled via basal stimulation with TNF-α (25 ng/mL) with or without nicotine (nAChR agonist) or bethanechol (mAChR agonist). Apoptosis, density (cells/cm2), TEER, lucifer yellow permeability, 70 kDa dextran transport, cell morphology, and IL-8 secretion were characterized.
Results: Primary intestinal epithelium demonstrates significant functional differences compared to immortalized cells, including increased barrier integrity, IL-8 expression, mucus production, and the presence of absorptive and secretory cells. Exposure to TNF-α impaired barrier integrity, increased apoptosis, altered morphology, and increased secretion of IL-8. Stimulation of nAChR with nicotine did not ameliorate TNF-α induced permeability nor alter 70 kDa dextran transport. However, stimulation of mAChR with bethanechol decreased transport of 70 kDa dextran but did not ameliorate TNF-α induced paracellular permeability.
Conclusions: A primary model of intestinal inflammation was evaluated, demonstrating nAChR or mAChR activation does not have the same protective effects compared to immortalized epithelium. Inclusion of other native stromal support cells are underway.
Keywords: Inflammation; Muscarinic; Nicotinic; Organoid; Tumor necrosis factor; mAChR; nAChR.
© Biomedical Engineering Society 2020.
Figures





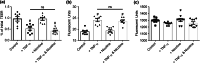


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

