Roles of cytokines and T cells in the pathogenesis of myasthenia gravis
- PMID: 33184844
- PMCID: PMC7874834
- DOI: 10.1111/cei.13546
Roles of cytokines and T cells in the pathogenesis of myasthenia gravis
Abstract
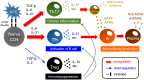
Myasthenia gravis (MG) is characterized by muscle weakness and fatigue caused by the presence of autoantibodies against the acetylcholine receptor (AChR) or the muscle-specific tyrosine kinase (MuSK). Activated T, B and plasma cells, as well as cytokines, play important roles in the production of pathogenic autoantibodies and the induction of inflammation at the neuromuscular junction in MG. Many studies have focused on the role of cytokines and lymphocytes in anti-AChR antibody-positive MG. Chronic inflammation mediated by T helper type 17 (Th17) cells, the promotion of autoantibody production from B cells and plasma cells by follicular Th (Tfh) cells and the activation of the immune response by dysfunction of regulatory T (Treg ) cells may contribute to the exacerbation of the MG pathogenesis. In fact, an increased number of Th17 cells and Tfh cells and dysfunction of Treg cells have been reported in patients with anti-AChR antibody-positive MG; moreover, the number of these cells was correlated with clinical parameters in patients with MG. Regarding cytokines, interleukin (IL)-17; a Th17-related cytokine, IL-21 (a Tfh-related cytokine), the B-cell-activating factor (BAFF; a B cell-related cytokine) and a proliferation-inducing ligand (APRIL; a B cell-related cytokine) have been reported to be up-regulated and associated with clinical parameters of MG. This review focuses on the current understanding of the involvement of cytokines and lymphocytes in the immunological pathogenesis of MG, which may lead to the development of novel therapies for this disease in the near future.
Keywords: B cell; cytokine; lymphocyte; myasthenia gravis; treatment.
© 2020 British Society for Immunology.
Conflict of interest statement
A. U., Y. O., M. Y. and T. I. report no competing interests. S. K. has received speaker honoraria from Alexion Pharmaceuticals, Teijin Pharmatheticals and CSL Behring. S. S. received personal fees from Alexion Pharmaceuticals, the Japan Blood Products Organization and Asahi Kasei Medical. H. M. has served as a paid consultant for Alexion Pharmaceuticals, argenx BVBA and Ra Pharmaceuticals and has received speaker honoraria from the Japan Blood Products Organization and research support from the Ministry of Health, Labour and Welfare, Japan. Y. N. has received speaker honoraria from Alexion Pharmaceuticals and Japan Blood Products Organization. K. U. has served as a paid consultant for argenx, Ra Pharmaceuticals and UCB Pharma and has received speaker honoraria from Alexion Pharmaceuticals and Japan Blood Products Organization.
Figures
References
-
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015; 14:1023–36. - PubMed
-
- Dalakas MC. Immunotherapy in myasthenia gravis in the era of biologics. Nat Rev Neurol 2019; 15:113–24. - PubMed
-
- Uzawa A, Kawaguchi N, Kanai T, Himuro K, Kuwabara S. Serum high mobility group box 1 is upregulated in myasthenia gravis. J Neurol Neurosurg Psychiatry 2015; 86:695–7. - PubMed
-
- Uzawa A, Kawaguchi N, Kanai T, Himuro K, Oda F, Kuwabara S. Increased serum peroxiredoxin 5 levels in myasthenia gravis. J Neuroimmunol 2015; 287:16–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

