Early experiences of nusinersen for the treatment of spinal muscular atrophy: Results from a large survey of patients and caregivers
- PMID: 33184859
- PMCID: PMC7986200
- DOI: 10.1002/mus.27116
Early experiences of nusinersen for the treatment of spinal muscular atrophy: Results from a large survey of patients and caregivers
Abstract
Background: This study aimed to examine the early experience of nusinersen for spinal muscular atrophy (SMA) from the patient and caregiver perspective.
Methods: A 54-item online survey was administered to adult patients and caregivers of pediatric patients diagnosed with SMA.
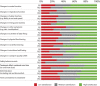
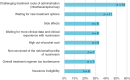
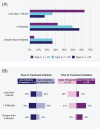
Results: Overall, respondents (56 patients and 45 caregivers) were satisfied with nusinersen. Satisfaction was highest on changes in energy, stamina, and motor function and lowest on treatment administration and overall time commitment. Differences were noted for treatment effect sustained over time as reported by adult patients vs caregivers reporting on behalf of pediatric patients. Respondents reported insurance approval as a key barrier to access, particularly among adult patients.
Conclusions: Despite therapeutic advances, there remain significant unmet needs for SMA. Challenges with administration and barriers to access potentially limit the number of patients treated or delay treatment. Continued efforts are needed to develop more treatment options and to improve access to treatments.
Keywords: SMA; burden; nusinersen; patient reported outcome; spinal muscular atrophy; unmet need.
© 2020 The Authors. Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
Study funded by Genentech Inc., A member of the Roche Group. Er Chen is an employee of GNE. Rupali Naik is a contracting employee of GNE. Josh M Noone, Daniel Buchenberger, Sarah M Whitmire and Rosalina Mills received research funding from GNE for the design and conduct of the research and writing of the paper. Stacy Dixon was contracted with GNE as clinical advisor of this project, no financial compensation was provided. Previously served as a paid member for an advisory board for Genentech. Additionally, have served as a paid member on several advisory boards for Biogen. William Arnold was contracted with GNE as clinical advisor of this project, no financial compensation was provided. Previously served as a consultant to Genentech and F. Hoffmann‐La Roche AG.
Figures
References
-
- Finkel RS, Mercuri E, Meyer OH, et al. Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28(3):197‐207. - PubMed
-
- Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103‐115. - PubMed
-
- Food and Drug Administration . FDA approves first drug for spinal muscular atrophy. 2016. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm534611.htm. Accessed June 20, 2019.
-
- Cure SMA . AveXis files for FDA approval of gene therapy for spinal muscular atrophy type I. 2018. http://www.curesma.org/news/avexis-fda-approval-type-i.html Accessed June 20, 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

