Inhibition of ATGL in adipose tissue ameliorates isoproterenol-induced cardiac remodeling by reducing adipose tissue inflammation
- PMID: 33185110
- PMCID: PMC7847076
- DOI: 10.1152/ajpheart.00737.2020
Inhibition of ATGL in adipose tissue ameliorates isoproterenol-induced cardiac remodeling by reducing adipose tissue inflammation
Abstract
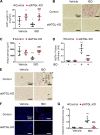
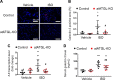
Following cardiac injury, increased adrenergic drive plays an important role in compensating for reduced cardiac function. However, chronic excess adrenergic stimulation can be detrimental to cardiac pathophysiology and can also affect other organs including adipose tissue, leading to increased lipolysis. Interestingly, inhibition of adipose triglyceride lipase (ATGL), a rate-limiting enzyme in lipolysis, in adipocytes ameliorates cardiac dysfunction in a heart failure model. Thus, we investigated whether inhibition of adipocyte ATGL can mitigate the adverse cardiac effects of chronic adrenergic stimulation and explored the underlying mechanisms. To do this, isoproterenol (ISO) was continuously administered to C57Bl/6N mice for 2 wk with or without an ATGL inhibitor (Atglistatin). We found that Atglistatin alleviated ISO-induced cardiac remodeling and reduced ISO-induced upregulation of galectin-3, a marker of activated macrophages and a potent inducer of fibrosis, in white adipose tissue (WAT), heart, and the circulation. To test whether the beneficial effects of Atglistatin occur via inhibition of adipocyte ATGL, adipocyte-specific ATGL knockout (atATGL-KO) mice were utilized for similar experiments. Subsequently, the same cardioprotective effects of atATGL-KO following ISO administration were observed. Furthermore, Atglistatin and atATGL-KO abolished ISO-induced galectin-3 secretion from excised WAT. We further demonstrated that activation of cardiac fibroblasts by the conditioned media of ISO-stimulated WAT is galectin-3-dependent. In conclusion, the inhibition of adipocyte ATGL ameliorated ISO-induced cardiac remodeling possibly by reducing galectin-3 secretion from adipose tissue. Thus, inhibition of adipocyte ATGL might be a potential target to prevent some of the adverse effects of chronic excess adrenergic drive.NEW & NOTEWORTHY The reduction of lipolysis by adipocyte ATGL inhibition ameliorates cardiac remodeling induced by chronic β-adrenergic stimulation likely via reducing galectin-3 secretion from adipose tissue. Our findings highlight that suppressing lipolysis in adipocytes may be a potential therapeutic target for patients with heart failure whose sympathetic nervous system is activated. Furthermore, galectin-3 might be involved in the mechanisms by which excessive lipolysis in adipose tissues influences remote cardiac pathologies and thus warrants further investigation.
Keywords: ATGL; adipose triglyceride lipase; cardiac remodeling; galectin-3; inflammation; isoproterenol.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Shimizu I, Yoshida Y, Katsuno T, Tateno K, Okada S, Moriya J, Yokoyama M, Nojima A, Ito T, Zechner R, Komuro I, Kobayashi Y, Minamino T. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab 15: 51–64, 2012. [Erratum in Cell Metab 2012 May 2; 15(5):787]. doi:10.1016/j.cmet.2011.12.006. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

