Influence of bacterial components on the developmental programming of enteric neurons
- PMID: 33185323
- PMCID: PMC7663985
- DOI: 10.14814/phy2.14611
Influence of bacterial components on the developmental programming of enteric neurons
Abstract
Background: Intestinal bacteria have been increasingly shown to be involved in early postnatal development. Previous work has shown that intestinal bacteria are necessary for the structural development and intrinsic function of the enteric nervous system in early postnatal life. Furthermore, colonization with a limited number of bacteria appears to be sufficient for the formation of a normal enteric nervous system. We tested the hypothesis that common bacterial components could influence the programming of developing enteric neurons.
Methods: The developmental programming of enteric neurons was studied by isolating enteric neural crest-derived cells from the fetal gut of C57Bl/6 mice at embryonic day 15.5. After the establishment of the cell line, cultured enteric neuronal precursors were exposed to increasing concentrations of a panel of bacterial components including lipopolysaccharide, flagellin, and components of peptidoglycan.
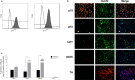
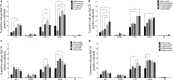
Key result: Exposure to bacterial components consistently affected proportions of enteric neuronal precursors that developed into nitrergic neurons. Furthermore, flagellin and D-gamma-Glu-mDAP were found to promote the development of serotonergic neurons. Proportions of dopaminergic neurons remained unchanged. Proliferation of neuronal precursor cells was significantly increased upon exposure to lipopolysaccharide and flagellin, while no significant changes were observed in the proportion of apoptotic neuronal precursors compared to baseline with exposure to any bacterial component.
Conclusions and interfaces: These findings suggest that bacterial components may influence the development of enteric neurons.
Keywords: bacterial components; development; enteric nervous system; enteric neural crest-derived cells.
© 2020 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors wish to declare no conflicts of interest relevant to the conducted research.
Figures
Similar articles
-
Neuronal Differentiation in Schwann Cell Lineage Underlies Postnatal Neurogenesis in the Enteric Nervous System.J Neurosci. 2015 Jul 8;35(27):9879-88. doi: 10.1523/JNEUROSCI.1239-15.2015. J Neurosci. 2015. PMID: 26156989 Free PMC article.
-
The alpha1 subunit of laminin-1 promotes the development of neurons by interacting with LBP110 expressed by neural crest-derived cells immunoselected from the fetal mouse gut.J Neurobiol. 1997 Aug;33(2):118-38. doi: 10.1002/(sici)1097-4695(199708)33:2<118::aid-neu2>3.0.co;2-5. J Neurobiol. 1997. PMID: 9240369
-
Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons.J Neurosci. 2011 Jun 15;31(24):8998-9009. doi: 10.1523/JNEUROSCI.6684-10.2011. J Neurosci. 2011. PMID: 21677183 Free PMC article.
-
Neurotrophin-3 in the development of the enteric nervous system.Prog Brain Res. 2004;146:243-63. doi: 10.1016/S0079-6123(03)46016-0. Prog Brain Res. 2004. PMID: 14699968 Review.
-
Pleiotropic effects of the bone morphogenetic proteins on development of the enteric nervous system.Dev Neurobiol. 2012 Jun;72(6):843-56. doi: 10.1002/dneu.22002. Dev Neurobiol. 2012. PMID: 22213745 Free PMC article. Review.
Cited by
-
MIAOME: Human microbiome affect the host epigenome.Comput Struct Biotechnol J. 2022 May 17;20:2455-2463. doi: 10.1016/j.csbj.2022.05.024. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35664224 Free PMC article.
-
Microbiota, mitochondria, and epigenetics in health and disease: converging pathways to solve the puzzle.Pflugers Arch. 2025 May;477(5):635-655. doi: 10.1007/s00424-025-03072-w. Epub 2025 Mar 20. Pflugers Arch. 2025. PMID: 40111427 Review.
References
-
- Akamatsu, W. , Fujihara, H. , Mitsuhashi, T. , Yano, M. , Shibata, S. , Hayakawa, Y. , …, Okano, H. (2005). The RNA‐binding protein HuD regulates neuronal cell identity and maturation. Proceedings of the National Academy of Sciences of the United States of America, 102(12), 4625–4630. 10.1073/pnas.0407523102 - DOI - PMC - PubMed
-
- Anitha, M. , Reichardt, F. , Tabatabavakili, S. , Nezami, B. G. , Chassaing, B. , Mwangi, S. , …, Srinivasan, S. (2016). Intestinal dysbiosis contributes to the delayed gastrointestinal transit in high‐fat diet fed mice. Cellular and Molecular Gastroenterology and Hepatology, 2(3), 328–339. 10.1016/j.jcmgh.2015.12.008 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

