Ubiquitin chains earmark GPCRs for BBSome-mediated removal from cilia
- PMID: 33185668
- PMCID: PMC7716378
- DOI: 10.1083/jcb.202003020
Ubiquitin chains earmark GPCRs for BBSome-mediated removal from cilia
Abstract
Regulated trafficking of G protein-coupled receptors (GPCRs) controls cilium-based signaling pathways. β-Arrestin, a molecular sensor of activated GPCRs, and the BBSome, a complex of Bardet-Biedl syndrome (BBS) proteins, are required for the signal-dependent exit of ciliary GPCRs, but the functional interplay between β-arrestin and the BBSome remains elusive. Here we find that, upon activation, ciliary GPCRs become tagged with ubiquitin chains comprising K63 linkages (UbK63) in a β-arrestin-dependent manner before BBSome-mediated exit. Removal of ubiquitin acceptor residues from the somatostatin receptor 3 (SSTR3) and from the orphan GPCR GPR161 demonstrates that ubiquitination of ciliary GPCRs is required for their regulated exit from cilia. Furthermore, targeting a UbK63-specific deubiquitinase to cilia blocks the exit of GPR161, SSTR3, and Smoothened (SMO) from cilia. Finally, ubiquitinated proteins accumulate in cilia of mammalian photoreceptors and Chlamydomonas cells when BBSome function is compromised. We conclude that Ub chains mark GPCRs and other unwanted ciliary proteins for recognition by the ciliary exit machinery.
© 2020 Shinde et al.
Figures
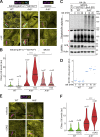
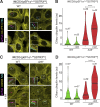
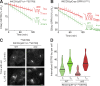
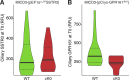
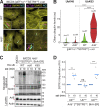
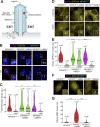
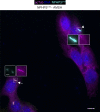


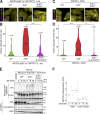
Comment in
-
Cilia Biology: You're It! Tagging Proteins for Ciliary Removal.Curr Biol. 2021 Jan 25;31(2):R80-R82. doi: 10.1016/j.cub.2020.11.030. Curr Biol. 2021. PMID: 33497637
References
-
- Chiang, A.P., Beck J.S., Yen H.J., Tayeh M.K., Scheetz T.E., Swiderski R.E., Nishimura D.Y., Braun T.A., Kim K.-Y.A., Huang J., et al. . 2006. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11). Proc. Natl. Acad. Sci. USA. 103:6287–6292. 10.1073/pnas.0600158103 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

