Probing the effect of aroma compounds on the hydrodynamic properties of mucin glycoproteins
- PMID: 33185715
- PMCID: PMC7701130
- DOI: 10.1007/s00249-020-01475-4
Probing the effect of aroma compounds on the hydrodynamic properties of mucin glycoproteins
Abstract
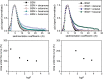
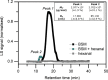
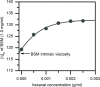
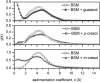
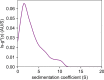
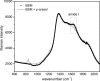
Aroma compounds are diverse low molecular weight organic molecules responsible for the flavour of food, medicines or cosmetics. Natural and artificial aroma compounds are manufactured and used by the industry to enhance the flavour and fragrance of products. While the low concentrations of aroma compounds present in food may leave no effect on the structural integrity of the mucosa, the effect of concentrated aroma volatiles is not well understood. At high concentrations, like those found in some flavoured products such as e-cigarettes, some aroma compounds are suggested to elicit a certain degree of change in the mucin glycoprotein network, depending on their functional group. These effects are particularly associated with carbonyl compounds such as aldehydes and ketones, but also phenols which may interact with mucin and other glycoproteins through other interaction mechanisms. This study demonstrates the formation of such interactions in vitro through the use of molecular hydrodynamics. Sedimentation velocity studies reveal that the strength of the carbonyl compound interaction is influenced by compound hydrophobicity, in which the more reactive short chain compounds show the largest increase in mucin-aroma sedimentation coefficients. By contrast, the presence of groups that increases the steric hindrance of the carbonyl group, such as ketones, produced a milder effect. The interaction effects were further demonstrated for hexanal using size exclusion chromatography light scattering (SEC-MALS) and intrinsic viscosity. In addition, phenolic aroma compounds were identified to reduce the sedimentation coefficient of mucin, which is consistent with interactions in the non-glycosylated mucin region.
Keywords: Aldehydes; Aroma; Hydrodynamics; Interactions; Mucin; Phenols.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Application of recent advances in hydrodynamic methods for characterising mucins in solution.Eur Biophys J. 2016 Jan;45(1):45-54. doi: 10.1007/s00249-015-1075-0. Epub 2015 Nov 23. Eur Biophys J. 2016. PMID: 26596272
-
Retention effect of human saliva on aroma release and respective contribution of salivary mucin and α-amylase.Food Res Int. 2014 Oct;64:424-431. doi: 10.1016/j.foodres.2014.07.013. Epub 2014 Jul 28. Food Res Int. 2014. PMID: 30011670
-
Submaxillary Mucin: its Effect on Aroma Release from Acidic Drinks and New Insight into the Effect of Aroma Compounds on its Macromolecular Integrity.Food Biophys. 2019;14(3):278-286. doi: 10.1007/s11483-019-09574-2. Epub 2019 Apr 15. Food Biophys. 2019. PMID: 31402849 Free PMC article.
-
Heterogeneity of high-molecular-weight human salivary mucins.Adv Dent Res. 2000 Dec;14:69-75. doi: 10.1177/08959374000140011101. Adv Dent Res. 2000. PMID: 11842927 Review.
-
Interactions of salivary mucins and saliva with food proteins: a review.Crit Rev Food Sci Nutr. 2020;60(1):64-83. doi: 10.1080/10408398.2018.1512950. Epub 2019 Jan 11. Crit Rev Food Sci Nutr. 2020. PMID: 30632771 Review.
Cited by
-
Analytical ultracentrifugation: still the gold standard that offers multiple solutions.Eur Biophys J. 2020 Dec;49(8):673-676. doi: 10.1007/s00249-020-01483-4. Eur Biophys J. 2020. PMID: 33211149
-
Smell compounds classification using UMAP to increase knowledge of odors and molecular structures linkages.PLoS One. 2021 May 28;16(5):e0252486. doi: 10.1371/journal.pone.0252486. eCollection 2021. PLoS One. 2021. PMID: 34048487 Free PMC article.
-
Advancing Discovery of Snail Mucins Function and Application.Front Bioeng Biotechnol. 2021 Oct 11;9:734023. doi: 10.3389/fbioe.2021.734023. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34708024 Free PMC article. Review.
References
-
- Arai S, Noguchi M, Yamashita M, et al. Studies on flavor components in soybean. Agric Biol Chem. 1970;34:1569–1573. doi: 10.1271/bbb1961.34.1569. - DOI
-
- Cohen SM, Eisenbrand G, Fukushima S, et al (2020) GRAS 29 Flavoring Substances. https://www.ift.org/news-and-publications/food-technology-magazine/issue.... Accessed 2 Jul 2020
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

