The Dysregulated Galectin Network Activates NF-κB to Induce Disease Markers and Matrix Degeneration in 3D Pellet Cultures of Osteoarthritic Chondrocytes
- PMID: 33185768
- PMCID: PMC7881967
- DOI: 10.1007/s00223-020-00774-4
The Dysregulated Galectin Network Activates NF-κB to Induce Disease Markers and Matrix Degeneration in 3D Pellet Cultures of Osteoarthritic Chondrocytes
Abstract
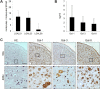
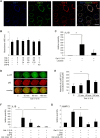
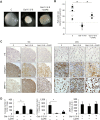
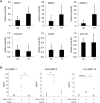
This work aimed to study the dysregulated network of galectins in OA chondrocyte pellets, and to assess whether their recently discovered activity as molecular switches of functional biomarkers results in degradation of extracellular matrix in vitro. Scaffold-free 3D pellet cultures were established of human OA chondrocytes. Expression and secretion of galectin(Gal)-1, -3, and -8 were monitored relative to 2D cultures or clinical tissue sections by RT-qPCR, immunohistochemistry and ELISAs. Exposure of 2D and 3D cultures to an in vivo-like galectin mixture (Gal-1 and Gal-8: 5 µg/ml, Gal-3: 1 µg/ml) was followed by the assessment of pellet size, immunohistochemical matrix staining, and/or quantification of MMP-1, -3, and -13. Application of inhibitors of NF-κB activation probed into the potential of intervening with galectin-induced matrix degradation. Galectin profiling revealed maintained dysregulation of Gal-1, -3, and -8 in pellet cultures, resembling the OA situation in situ. The presence of the galectin mixture promoted marked reduction of pellet size and loss of collagen type II-rich extracellular matrix, accompanied by the upregulation of MMP-1, -3, and -13. Inhibition of p65-phosphorylation by caffeic acid phenethyl ester effectively alleviated the detrimental effects of galectins, resulting in downregulated MMP secretion, reduced matrix breakdown and augmented pellet size. This study suggests that the dysregulated galectin network in OA cartilage leads to extracellular matrix breakdown, and provides encouraging evidence of the feasible inhibition of galectin-triggered activities. OA chondrocyte pellets have the potential to serve as in vitro disease model for further studies on galectins in OA onset and progression.
Keywords: 3D cell culture; Chondrocytes; Galectin; Lectin; NF-κB; Osteoarthritis.
Conflict of interest statement
K. M. Pichler, D. Weinmann, S. Schmidt, B. Kubista, R. Lass, L. Martelanz, J. Alphonsus, R. Windhager, H.-J. Gabius, S. Toegel have no conflicts of interest to disclose.
Figures

Similar articles
-
Galectin network in osteoarthritis: galectin-4 programs a pathogenic signature of gene and effector expression in human chondrocytes in vitro.Histochem Cell Biol. 2022 Feb;157(2):139-151. doi: 10.1007/s00418-021-02053-1. Epub 2021 Nov 30. Histochem Cell Biol. 2022. PMID: 34846578 Free PMC article.
-
Galectin-3 Induces a Pro-degradative/inflammatory Gene Signature in Human Chondrocytes, Teaming Up with Galectin-1 in Osteoarthritis Pathogenesis.Sci Rep. 2016 Dec 16;6:39112. doi: 10.1038/srep39112. Sci Rep. 2016. PMID: 27982117 Free PMC article.
-
Galectin-1 Couples Glycobiology to Inflammation in Osteoarthritis through the Activation of an NF-κB-Regulated Gene Network.J Immunol. 2016 Feb 15;196(4):1910-21. doi: 10.4049/jimmunol.1501165. Epub 2016 Jan 20. J Immunol. 2016. PMID: 26792806
-
The mechanistic role of curcumin on matrix metalloproteinases in osteoarthritis.Fitoterapia. 2024 Apr;174:105870. doi: 10.1016/j.fitote.2024.105870. Epub 2024 Feb 28. Fitoterapia. 2024. PMID: 38423225 Review.
-
Towards a Better Understanding of the Relationships between Galectin-7, p53 and MMP-9 during Cancer Progression.Biomolecules. 2021 Jun 14;11(6):879. doi: 10.3390/biom11060879. Biomolecules. 2021. PMID: 34198494 Free PMC article. Review.
Cited by
-
Caffeic acid phenethyl ester attenuates osteoarthritis progression by activating NRF2/HO‑1 and inhibiting the NF‑κB signaling pathway.Int J Mol Med. 2022 Nov;50(5):134. doi: 10.3892/ijmm.2022.5190. Epub 2022 Sep 14. Int J Mol Med. 2022. PMID: 36102306 Free PMC article.
-
Anti-Inflammatory and Pro-Regenerative Effects of Hyaluronan-Chitlac Mixture in Human Dermal Fibroblasts: A Skin Ageing Perspective.Polymers (Basel). 2022 Apr 29;14(9):1817. doi: 10.3390/polym14091817. Polymers (Basel). 2022. PMID: 35566988 Free PMC article.
-
Galectin network in osteoarthritis: galectin-4 programs a pathogenic signature of gene and effector expression in human chondrocytes in vitro.Histochem Cell Biol. 2022 Feb;157(2):139-151. doi: 10.1007/s00418-021-02053-1. Epub 2021 Nov 30. Histochem Cell Biol. 2022. PMID: 34846578 Free PMC article.
-
The degenerated glenohumeral joint.Bone Joint Res. 2024 Oct 21;13(10):596-610. doi: 10.1302/2046-3758.1310.BJR-2024-0026.R1. Bone Joint Res. 2024. PMID: 39428110 Free PMC article.
-
What is the Sugar Code?Chembiochem. 2022 Jul 5;23(13):e202100327. doi: 10.1002/cbic.202100327. Epub 2021 Sep 22. Chembiochem. 2022. PMID: 34496130 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

