Aberrant expression of junctional adhesion molecule-A contributes to the malignancy of cervical adenocarcinoma by interaction with poliovirus receptor/CD155
- PMID: 33185939
- PMCID: PMC7893988
- DOI: 10.1111/cas.14734
Aberrant expression of junctional adhesion molecule-A contributes to the malignancy of cervical adenocarcinoma by interaction with poliovirus receptor/CD155
Abstract
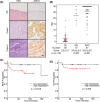
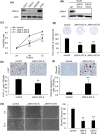
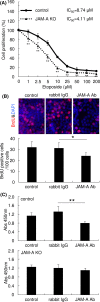
Recent studies have shown that aberrant expression of tight junction proteins (TJP) contributes to malignant potential of various cancers. In the present study, we investigated the expression of junctional adhesion molecule-A (JAM-A), one of the transmembrane TJP, in uterine cervical adenocarcinoma and the significance of its expression for malignancy. Immunohistochemistry on human surgical specimens showed that JAM-A was aberrantly expressed in neoplastic regions including adenocarcinoma in situ (AIS). Knockout of JAM-A significantly suppressed cell proliferation and colony-forming and migration abilities. We also showed that an antibody specific to an extracellular region of JAM-A reduced cell proliferation ability and that loss of JAM-A increased drug sensitivity of cervical adenocarcinoma cells. Based on a comprehensive proteome analysis, we found that poliovirus receptor (PVR/CD155) was regulated by JAM-A and formed a physical interaction with JAM-A. In human surgical specimens, PVR/CD155 expression was significantly correlated with some clinicopathological features and prognosis of cervical adenocarcinoma. Interestingly, most of the PVR/CD155-positive cases expressed a high level of JAM-A, and patients with the expression pattern of PVR/CD155 positive/JAM-A high had significantly shorter periods of relapse-free survival (P = .00964) and overall survival (P = .0204) than those for the other patients. Our observations suggest that aberrant expression of JAM-A promotes malignancy of uterine cervical adenocarcinoma by regulation of PVR/CD155, and JAM-A is therefore a potential therapeutic target for this malignancy.
Keywords: CD155; junctional adhesion molecule-A; poliovirus receptor; therapeutic target; tight junction protein; uterine cervical adenocarcinoma.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Multilayered proteomics reveals that JAM-A promotes breast cancer progression via regulation of amino acid transporter LAT1.Cancer Sci. 2024 Sep;115(9):3153-3168. doi: 10.1111/cas.16259. Epub 2024 Jun 29. Cancer Sci. 2024. PMID: 38943512 Free PMC article.
-
Analysis of the expression and localization of tight junction transmembrane proteins, claudin-1, -4, -7, occludin and JAM-A, in human cervical adenocarcinoma.Histol Histopathol. 2016 Aug;31(8):921-31. doi: 10.14670/HH-11-729. Epub 2016 Feb 5. Histol Histopathol. 2016. PMID: 26847087
-
Elevated expression of JAM-A promotes neoplastic properties of lung adenocarcinoma.Cancer Sci. 2017 Nov;108(11):2306-2314. doi: 10.1111/cas.13385. Epub 2017 Sep 18. Cancer Sci. 2017. PMID: 28837251 Free PMC article.
-
Dysregulation of JAM-A plays an important role in human tumor progression.Int J Clin Exp Pathol. 2014 Sep 15;7(10):7242-8. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25400822 Free PMC article. Review.
-
CD155, an onco-immunologic molecule in human tumors.Cancer Sci. 2017 Oct;108(10):1934-1938. doi: 10.1111/cas.13324. Epub 2017 Aug 18. Cancer Sci. 2017. PMID: 28730595 Free PMC article. Review.
Cited by
-
Multilayered proteomics reveals that JAM-A promotes breast cancer progression via regulation of amino acid transporter LAT1.Cancer Sci. 2024 Sep;115(9):3153-3168. doi: 10.1111/cas.16259. Epub 2024 Jun 29. Cancer Sci. 2024. PMID: 38943512 Free PMC article.
-
Aberrant expression of claudin-6 contributes to malignant potentials and drug resistance of cervical adenocarcinoma.Cancer Sci. 2022 Apr;113(4):1519-1530. doi: 10.1111/cas.15284. Epub 2022 Feb 13. Cancer Sci. 2022. PMID: 35100472 Free PMC article.
-
The Roles of Junctional Adhesion Molecules (JAMs) in Cell Migration.Front Cell Dev Biol. 2022 Mar 9;10:843671. doi: 10.3389/fcell.2022.843671. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35356274 Free PMC article. Review.
-
Prognostic and clinicopathological significance of CD155 expression in cancer patients: a meta-analysis.World J Surg Oncol. 2022 Oct 29;20(1):351. doi: 10.1186/s12957-022-02813-w. World J Surg Oncol. 2022. PMID: 36309698 Free PMC article. Review.
-
Bispecific antibody targeting CD155 mediates T-cell immunotherapy against human gynecological malignancies.Invest New Drugs. 2025 Apr;43(2):318-327. doi: 10.1007/s10637-025-01529-4. Epub 2025 Apr 15. Invest New Drugs. 2025. PMID: 40232354

