Cisplatin Prodrug-Loaded Nanoparticles Based on Physalis Mottle Virus for Cancer Therapy
- PMID: 33186039
- PMCID: PMC8087187
- DOI: 10.1021/acs.molpharmaceut.0c00834
Cisplatin Prodrug-Loaded Nanoparticles Based on Physalis Mottle Virus for Cancer Therapy
Abstract
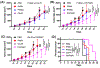
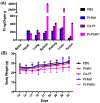
Nanoparticle-based prodrugs offer an effective strategy to improve the safety and delivery of small-molecule therapeutics while reducing the risk of drug resistance. Here, we conjugated a maleimide-functionalized cisplatin prodrug containing Pt(IV) to the internal and/or external surface of virus-like particles (VLPs) derived from Physalis mottle virus (PhMV) to develop a pH-sensitive drug delivery system. The internally loaded and PEGylated VLPs (Pt-PhMVCy5.5-PEG) were taken up efficiently by cancer cells where they released platinum, presumably as a reduced, DNA-reactive Pt(II) complex, rapidly under acidic conditions in vitro (>80% in 30 h). The efficacy of the VLP-based drug delivery system was demonstrated against a panel of cancer cell lines, including cell lines resistant to platinum therapy. Furthermore, Pt-PhMVCy5.5-PEG successfully inhibited the growth of xenograft MDA-MB-231 breast tumors in vivo and significantly prolonged the survival of mice compared to free cisplatin and cisplatin-maleimide. Pt-PhMVCy5.5-PEG therefore appears promising as a prodrug to overcome the limitations of conventional platinum-based drugs for cancer therapy.
Keywords: cisplatin resistance; nanomedicine; pH-responsive drug delivery; plant virus-like nanoparticles; prodrug.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Doxorubicin-Loaded Physalis Mottle Virus Particles Function as a pH-Responsive Prodrug Enabling Cancer Therapy.Biotechnol J. 2020 Dec;15(12):e2000077. doi: 10.1002/biot.202000077. Epub 2020 Oct 8. Biotechnol J. 2020. PMID: 32918857 Free PMC article.
-
Physalis Mottle Virus-Like Particles as Nanocarriers for Imaging Reagents and Drugs.Biomacromolecules. 2017 Dec 11;18(12):4141-4153. doi: 10.1021/acs.biomac.7b01196. Epub 2017 Nov 16. Biomacromolecules. 2017. PMID: 29144726 Free PMC article.
-
Biodegradable and pH-Responsive Acetalated Dextran (Ac-Dex) Nanoparticles for NIR Imaging and Controlled Delivery of a Platinum-Based Prodrug into Cancer Cells.Mol Pharm. 2019 May 6;16(5):2083-2094. doi: 10.1021/acs.molpharmaceut.9b00058. Epub 2019 Apr 1. Mol Pharm. 2019. PMID: 30901218
-
Prodrugs in combination with nanocarriers as a strategy for promoting antitumoral efficiency.Future Med Chem. 2019 Aug;11(16):2131-2150. doi: 10.4155/fmc-2018-0388. Future Med Chem. 2019. PMID: 31538520 Review.
-
Emerging platinum(IV) prodrug nanotherapeutics: A new epoch for platinum-based cancer therapy.J Control Release. 2023 Sep;361:819-846. doi: 10.1016/j.jconrel.2023.08.035. Epub 2023 Aug 23. J Control Release. 2023. PMID: 37597809 Review.
Cited by
-
Targeted Cancer Therapy via pH-Functionalized Nanoparticles: A Scoping Review of Methods and Outcomes.Gels. 2022 Apr 11;8(4):232. doi: 10.3390/gels8040232. Gels. 2022. PMID: 35448133 Free PMC article.
-
Potential Application of Self-Assembled Peptides and Proteins in Breast Cancer and Cervical Cancer.Int J Mol Sci. 2023 Dec 2;24(23):17056. doi: 10.3390/ijms242317056. Int J Mol Sci. 2023. PMID: 38069380 Free PMC article. Review.
-
Role of Nanotechnology and Their Perspectives in the Treatment of Kidney Diseases.Front Genet. 2022 Jan 5;12:817974. doi: 10.3389/fgene.2021.817974. eCollection 2021. Front Genet. 2022. PMID: 35069707 Free PMC article. Review.
-
Bacterial expression systems based on Tymovirus-like particles for the presentation of vaccine antigens.Front Microbiol. 2023 Mar 23;14:1154990. doi: 10.3389/fmicb.2023.1154990. eCollection 2023. Front Microbiol. 2023. PMID: 37032851 Free PMC article.
-
Development of a Virus-Like Particle-Based Anti-HER2 Breast Cancer Vaccine.Cancers (Basel). 2021 Jun 10;13(12):2909. doi: 10.3390/cancers13122909. Cancers (Basel). 2021. PMID: 34200802 Free PMC article.
References
-
- Cancer Facts & Figures. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-... (03/26/2020),
-
- Pushpakom S; Iorio F; Eyers PA; Escott KJ; Hopper S; Wells A; Doig A; Guilliams T; Latimer J; McNamee C; Norris A; Sanseau P; Cavalla D; Pirmohamed M Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discovery 2018, 18, 41–58. - PubMed
-
- Ghosh S Cisplatin: The first metal based anticancer drug. Bioorg. Chem 2019, 88, 102925. - PubMed
-
- Wang D; Lippard SJ Cellular processing of platinum anticancer drugs. Nat Rev Drug Discovery 2005, 4, 307–320. - PubMed
-
- Galluzzi L; Senovilla L; Vitale I; Michels J; Martins I; Kepp O; Castedo M; Kroemer G Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

