The synapse in traumatic brain injury
- PMID: 33186462
- PMCID: PMC7880663
- DOI: 10.1093/brain/awaa321
The synapse in traumatic brain injury
Abstract
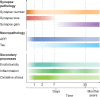
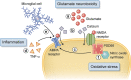
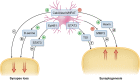
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide and is a risk factor for dementia later in life. Research into the pathophysiology of TBI has focused on the impact of injury on the neuron. However, recent advances have shown that TBI has a major impact on synapse structure and function through a combination of the immediate mechanical insult and the ensuing secondary injury processes, leading to synapse loss. In this review, we highlight the role of the synapse in TBI pathophysiology with a focus on the confluence of multiple secondary injury processes including excitotoxicity, inflammation and oxidative stress. The primary insult triggers a cascade of events in each of these secondary processes and we discuss the complex interplay that occurs at the synapse. We also examine how the synapse is impacted by traumatic axonal injury and the role it may play in the spread of tau after TBI. We propose that astrocytes play a crucial role by mediating both synapse loss and recovery. Finally, we highlight recent developments in the field including synapse molecular imaging, fluid biomarkers and therapeutics. In particular, we discuss advances in our understanding of synapse diversity and suggest that the new technology of synaptome mapping may prove useful in identifying synapses that are vulnerable or resistant to TBI.
Keywords: astrocyte; inflammation; microglia; synaptome; synaptopathy.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
References
-
- Anderson KJ, Scheff SW, DeKosky ST.. Reactive synaptogenesis in hippocampal area CA1 of aged and young adult rats. J Comp Neurol 1986; 252: 374–84. - PubMed
-
- Ansari MA, Roberts KN, Scheff SW.. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J Neurotrauma 2008; 25: 513–26. - PubMed
-
- Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM.. White matter involvement after TBI: clues to axon and myelin repair capacity. Exp Neurol 2016; 275: 328–33. - PubMed

