Measuring the Contributions of Basal Laminar Deposit and Bruch's Membrane in Age-Related Macular Degeneration
- PMID: 33186466
- PMCID: PMC7671869
- DOI: 10.1167/iovs.61.13.19
Measuring the Contributions of Basal Laminar Deposit and Bruch's Membrane in Age-Related Macular Degeneration
Abstract
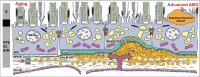
Purpose: Basal laminar deposit (BLamD) is a consistent finding in age-related macular degeneration (AMD). We quantified BLamD thickness, appearance, and topography in eyes of aged donors with and without AMD and evaluated its relationship to other components of the retinal pigment epithelium-basal lamina/Bruch's membrane (RPE-BL-BrM) complex.
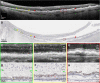
Methods: Donor eyes (n = 132) were classified as normal (n = 54), early to intermediate AMD (n = 24), geographic atrophy (GA; n = 13), and neovascular AMD (NV; n = 41). In high-resolution histology, we assessed RPE, BLamD, and BrM thicknesses and phenotypes at 3309 predefined locations in the central (foveal and perifovea) and superior (perifoveal) sections. Pre-mortem optical coherence tomography (OCT) imaging of a 90-year-old woman was compared to postmortem histopathology.
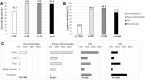
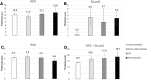
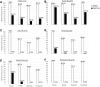
Results: In non-atrophic areas of AMD eyes, the RPE-BLamD is thick (normal = 13.7 µm, early-intermediate = 16.8 µm, GA = 17.4 µm, NV = 18.7 µm), because the BLamD is thick (normal = 0.3 µm, early-intermediate = 5.5 µm, GA = 4.1 µm, NV = 5.3 µm). RPE layer thickness is similar across these stages. Disease-associated variants of BLamD (thick, late, basal mounds) cluster subfoveally. A thick BLamD is visible on OCT as a hyporeflective split in the RPE-BL-BrM complex. BrM is thin (3.5 µm) in NV (normal = 4.2 µm, early to intermediate = 4.4 µm, and GA = 4.2 µm).
Conclusions: The RPE-BL-BrM complex is thick in AMD, driven by the accumulation and expansion of BLamD rather than expansion of either three-layer BrM, RPE-BL, or RPE. BLamD is clinically appreciable by OCT in some patients as a non-neovascular "split RPE-BL-BrM complex" or "double-layer sign." BLamD may contribute toward the formation and progression of high-risk drusen yet also exhibit protective properties.
Conflict of interest statement
Disclosure:
Figures



References
-
- Flaxman SR, Bourne RRA, Resnikoff S, et al.. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017; 5: e1221–e1234. - PubMed
-
- Comparison of Age-related Macular Degeneration Treatments Trials Research Group, Maguire MG, Martin DF, et al.. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016; 123: 1751–1761. - PMC - PubMed
-
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013; 309: 2005–2015. - PubMed
-
- Staurenghi G, Sadda S, Chakravarthy U, Spaide RF, International Nomenclature for Optical Coherence Tomography (IN OCT) Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN*OCT consensus. Ophthalmology. 2014; 121: 1572–1578. - PubMed
-
- Gass JDM. Stereoscopic atlas of macular diseases: diagnosis and treatment. St. Louis, MO: Mosby; 1997.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

