Polypill with or without Aspirin in Persons without Cardiovascular Disease
- PMID: 33186492
- PMCID: PMC7116860
- DOI: 10.1056/NEJMoa2028220
Polypill with or without Aspirin in Persons without Cardiovascular Disease
Abstract
Background: A polypill comprising statins, multiple blood-pressure-lowering drugs, and aspirin has been proposed to reduce the risk of cardiovascular disease.
Methods: Using a 2-by-2-by-2 factorial design, we randomly assigned participants without cardiovascular disease who had an elevated INTERHEART Risk Score to receive a polypill (containing 40 mg of simvastatin, 100 mg of atenolol, 25 mg of hydrochlorothiazide, and 10 mg of ramipril) or placebo daily, aspirin (75 mg) or placebo daily, and vitamin D or placebo monthly. We report here the outcomes for the polypill alone as compared with matching placebo, for aspirin alone as compared with matching placebo, and for the polypill plus aspirin as compared with double placebo. For the polypill-alone and polypill-plus-aspirin comparisons, the primary outcome was death from cardiovascular causes, myocardial infarction, stroke, resuscitated cardiac arrest, heart failure, or revascularization. For the aspirin comparison, the primary outcome was death from cardiovascular causes, myocardial infarction, or stroke. Safety was also assessed.
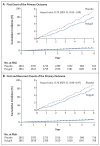
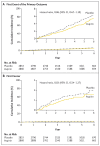
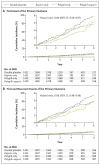
Results: A total of 5713 participants underwent randomization, and the mean follow-up was 4.6 years. The low-density lipoprotein cholesterol level was lower by approximately 19 mg per deciliter and systolic blood pressure was lower by approximately 5.8 mm Hg with the polypill and with combination therapy than with placebo. The primary outcome for the polypill comparison occurred in 126 participants (4.4%) in the polypill group and in 157 (5.5%) in the placebo group (hazard ratio, 0.79; 95% confidence interval [CI], 0.63 to 1.00). The primary outcome for the aspirin comparison occurred in 116 participants (4.1%) in the aspirin group and in 134 (4.7%) in the placebo group (hazard ratio, 0.86; 95% CI, 0.67 to 1.10). The primary outcome for the polypill-plus-aspirin comparison occurred in 59 participants (4.1%) in the combined-treatment group and in 83 (5.8%) in the double-placebo group (hazard ratio, 0.69; 95% CI, 0.50 to 0.97). The incidence of hypotension or dizziness was higher in groups that received the polypill than in their respective placebo groups.
Conclusions: Combined treatment with a polypill plus aspirin led to a lower incidence of cardiovascular events than did placebo among participants without cardiovascular disease who were at intermediate cardiovascular risk. (Funded by the Wellcome Trust and others; TIPS-3 ClinicalTrials.gov number, NCT01646437.).
Copyright © 2020 Massachusetts Medical Society.
Figures

Comment in
-
A one-size-fits-all polypill strategy for primary prevention in the era of precision medicine?Eur Heart J. 2021 Feb 11;42(6):561-562. doi: 10.1093/eurheartj/ehaa1064. Eur Heart J. 2021. PMID: 33471085 No abstract available.
-
Polypills - A Central Strategy for Improving Cardiovascular Health.N Engl J Med. 2021 Jan 21;384(3):288-289. doi: 10.1056/NEJMe2033310. N Engl J Med. 2021. PMID: 33471983 No abstract available.
-
In at-risk patients without CVD, polypill plus aspirin reduced a composite of major CV events at 4.6 y.Ann Intern Med. 2021 Apr;174(4):JC41. doi: 10.7326/ACPJ202104200-041. Epub 2021 Apr 6. Ann Intern Med. 2021. PMID: 33819061
-
Polypill in Persons without Cardiovascular Disease.N Engl J Med. 2021 Apr 29;384(17):1673-1674. doi: 10.1056/NEJMc2102972. N Engl J Med. 2021. PMID: 33913648 No abstract available.
-
Polypill in Persons without Cardiovascular Disease.N Engl J Med. 2021 Apr 29;384(17):1674. doi: 10.1056/NEJMc2102972. N Engl J Med. 2021. PMID: 33913649 No abstract available.
-
Polypill in Persons without Cardiovascular Disease.N Engl J Med. 2021 Apr 29;384(17):1674-1675. doi: 10.1056/NEJMc2102972. N Engl J Med. 2021. PMID: 33913650 No abstract available.
-
Polypill in Persons without Cardiovascular Disease.N Engl J Med. 2021 Apr 29;384(17):1675. doi: 10.1056/NEJMc2102972. N Engl J Med. 2021. PMID: 33913651 No abstract available.
-
Polypill in Persons without Cardiovascular Disease.N Engl J Med. 2021 Apr 29;384(17):1675-1676. doi: 10.1056/NEJMc2102972. N Engl J Med. 2021. PMID: 33913652 No abstract available.
-
Eine Monsterpille zur Prophylaxe bei Herzgesunden.MMW Fortschr Med. 2021 May;163(9):24-25. doi: 10.1007/s15006-021-9922-7. MMW Fortschr Med. 2021. PMID: 33961242 Free PMC article. German. No abstract available.
References
-
- Dagenais GR, Leong DP, Rangarajan S, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020;395:785–94. - PubMed
-
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–94. - PMC - PubMed
-
- The Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a metaanalysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
