CCR5 inhibition in critical COVID-19 patients decreases inflammatory cytokines, increases CD8 T-cells, and decreases SARS-CoV2 RNA in plasma by day 14
- PMID: 33186704
- PMCID: PMC7654230
- DOI: 10.1016/j.ijid.2020.10.101
CCR5 inhibition in critical COVID-19 patients decreases inflammatory cytokines, increases CD8 T-cells, and decreases SARS-CoV2 RNA in plasma by day 14
Abstract
Objective: Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is now a global pandemic. Emerging results indicate a dysregulated immune response. Given the role of CCR5 in immune cell migration and inflammation, we investigated the impact of CCR5 blockade via the CCR5-specific antibody leronlimab on clinical, immunological, and virological parameters in severe COVID-19 patients.
Methods: In March 2020, 10 terminally ill, critical COVID-19 patients received two doses of leronlimab via individual emergency use indication. We analyzed changes in clinical presentation, immune cell populations, inflammation, as well as SARS-CoV-2 plasma viremia before and 14 days after treatment.
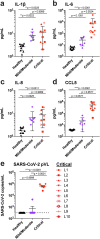
Results: Over the 14-day study period, six patients survived, two were extubated, and one discharged. We observed complete CCR5 receptor occupancy in all donors by day 7. Compared with the baseline, we observed a concomitant statistically significant reduction in plasma IL-6, restoration of the CD4/CD8 ratio, and resolution of SARS-CoV2 plasma viremia (pVL). Furthermore, the increase in the CD8 percentage was inversely correlated with the reduction in pVL (r = -0.77, p = 0.0013).
Conclusions: Our study design precludes clinical efficacy inferences but the results implicate CCR5 as a therapeutic target for COVID-19 and they form the basis for ongoing randomized clinical trials.
Keywords: CCR5; COVID-19; Immunotherapy; Leronlimab; Plasma viral load.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Chen L., Zhang Q., Yu C., Wang F., Kong X. Functional roles of CCL5/RANTES in liver disease. Liver Res. 2020;4:28–34.
-
- Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;8:970–979. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

