Spike Glycoprotein-Mediated Entry of SARS Coronaviruses
- PMID: 33187074
- PMCID: PMC7696831
- DOI: 10.3390/v12111289
Spike Glycoprotein-Mediated Entry of SARS Coronaviruses
Abstract
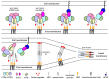
Severe acute respiratory syndrome coronavirus (SARS-CoV) and SARS-CoV-2 are enveloped, positive-sense, single-stranded RNA viruses and causes of epidemic diseases that have resulted in public health emergencies worldwide. Angiotensin-converting enzyme 2 (ACE2) is the receptor that allows the entry of these two viruses into host cells, a key step in the life cycle of the pathogens. The characterization of the interactions of ACE2 with the viral spike glycoproteins and structural studies of the ACE2-binding-induced conformational changes in the viral spike glycoproteins have furthered our understanding of the entry processes of these two viruses, and these studies provide useful information that will facilitate the development of antiviral agents and vaccines to control the diseases.
Keywords: COVID-19; SARS coronaviruses; conformational change; entry; membrane fusion; receptor binding; spike glycoprotein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

