Modulation of the Neuroprotective and Anti-inflammatory Activities of the Flavonol Fisetin by the Transition Metals Iron and Copper
- PMID: 33187316
- PMCID: PMC7696754
- DOI: 10.3390/antiox9111113
Modulation of the Neuroprotective and Anti-inflammatory Activities of the Flavonol Fisetin by the Transition Metals Iron and Copper
Abstract
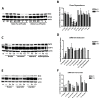
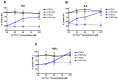
Alterations occur in the homeostasis of the transition metals iron (Fe2+) and copper (Cu2+) during aging and these are further amplified in neurodegenerative diseases, including Alzheimer's disease (AD). These observations suggest that the most effective drug candidates for AD might be those that can reduce these alterations. The flavonoid fisetin has both neuroprotective and anti-inflammatory activity both in vitro and in vivo and can bind both iron and copper suggesting that its chelating activity might play a role in its beneficial effects. To test this idea, the effects of iron and copper on both the neuroprotective and anti-inflammatory activities of fisetin were examined. It is shown that while fisetin can reduce the potentiation of cell death by iron and copper in response to treatments that lower glutathione levels, it is much less effective when the metals are combined with other inducers of oxidative stress. In addition, iron but not copper reduces the anti-inflammatory effects of fisetin in a dose-dependent manner. These effects correlate with the ability of iron but not copper to block the induction of the antioxidant transcription factor, Nrf2, by fisetin. In contrast, although the flavanone sterubin also binds iron, the metal has no effect on sterubin's ability to induce Nrf2 or protect cells from toxic or pro-inflammatory insults. Together, these results suggest that while iron and copper binding could contribute to the beneficial effects of neuroprotective compounds in the context of neurodegenerative diseases, the consequences of this binding need to be fully examined for each compound.
Keywords: Alzheimer’s disease; Nrf2; ferroptosis; glutathione; oxidative stress; oxytosis; sterubin.
Conflict of interest statement
The author declares no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

