Advances in Optical Detection of Human-Associated Pathogenic Bacteria
- PMID: 33187331
- PMCID: PMC7696695
- DOI: 10.3390/molecules25225256
Advances in Optical Detection of Human-Associated Pathogenic Bacteria
Abstract
Bacterial infection is a global burden that results in numerous hospital visits and deaths annually. The rise of multi-drug resistant bacteria has dramatically increased this burden. Therefore, there is a clinical need to detect and identify bacteria rapidly and accurately in their native state or a culture-free environment. Current diagnostic techniques lack speed and effectiveness in detecting bacteria that are culture-negative, as well as options for in vivo detection. The optical detection of bacteria offers the potential to overcome these obstacles by providing various platforms that can detect bacteria rapidly, with minimum sample preparation, and, in some cases, culture-free directly from patient fluids or even in vivo. These modalities include infrared, Raman, and fluorescence spectroscopy, along with optical coherence tomography, interference, polarization, and laser speckle. However, these techniques are not without their own set of limitations. This review summarizes the strengths and weaknesses of utilizing each of these optical tools for rapid bacteria detection and identification.
Keywords: OCT; Raman; bacterial infection; fluorescence; infrared; optical detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
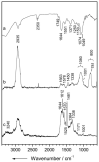



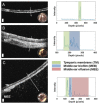
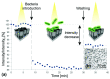
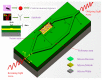

References
-
- Centers for Disease Control and Prevention About Antibiotic Resistance. [(accessed on 3 July 2019)];2013 Available online: https://www.cdc.gov/drugresistance/about.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

