Characterization of Mesenchymal Stem Cell Differentiation within Miniaturized 3D Scaffolds through Advanced Microscopy Techniques
- PMID: 33187392
- PMCID: PMC7696107
- DOI: 10.3390/ijms21228498
Characterization of Mesenchymal Stem Cell Differentiation within Miniaturized 3D Scaffolds through Advanced Microscopy Techniques
Abstract
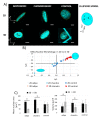
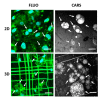
Three-dimensional culture systems and suitable substrates topographies demonstrated to drive stem cell fate in vitro by mechanical conditioning. For example, the Nichoid 3D scaffold remodels stem cells and shapes nuclei, thus promoting stem cell expansion and stemness maintenance. However, the mechanisms involved in force transmission and in biochemical signaling at the basis of fate determination are not yet clear. Among the available investigation systems, confocal fluorescence microscopy using fluorescent dyes enables the observation of cell function and shape at the subcellular scale in vital and fixed conditions. Contrarily, nonlinear optical microscopy techniques, which exploit multi-photon processes, allow to study cell behavior in vital and unlabeled conditions. We apply confocal fluorescence microscopy, coherent anti-Stokes Raman scattering (CARS), and second harmonic generation (SHG) microscopy to characterize the phenotypic expression of mesenchymal stem cells (MSCs) towards adipogenic and chondrogenic differentiation inside Nichoid scaffolds, in terms of nuclear morphology and specific phenotypic products, by comparing these techniques. We demonstrate that the Nichoid maintains a rounded nuclei during expansion and differentiation, promoting MSCs adipogenic differentiation while inhibiting chondrogenesis. We show that CARS and SHG techniques are suitable for specific estimation of the lipid and collagenous content, thus overcoming the limitations of using unspecific fluorescent probes.
Keywords: 3D culture; CARS; SHG; nonlinear microscopy; stem cell differentiation.
Conflict of interest statement
M.T.R., G.C. and R.O. are co-founders of a university spin-off company, MOAB S.r.l., and hold shares.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

