Identification of a novel subpopulation of Caspase-4 positive non-small cell lung Cancer patients
- PMID: 33187551
- PMCID: PMC7664047
- DOI: 10.1186/s13046-020-01754-0
Identification of a novel subpopulation of Caspase-4 positive non-small cell lung Cancer patients
Abstract
Background: Therapy/prognosis of Non-Small Cell Lung Cancer (NSCLC) patients are strongly related to gene alteration/s or protein expression. However, more than 50% of NSCLC patients are negative to key drugable biomarkers.
Methods: We used human samples of NSCLC and mouse models of lung adenocarcinoma.
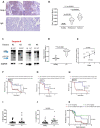
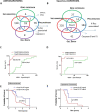
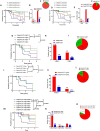
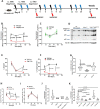
Results: We showed that caspase-4 was highly present in the tumor mass compared to non-cancerous human tissues. Interestingly, the orthologue murine caspase-11 promoted lung carcinogenesis in mice. Carcinogen-exposed caspase-11 knockout mice had lower tumor lesions than wild type mice, due to the relevance of caspase-11 in the structural lung cell as demonstrated by bone marrow transplantation and adoptive transfer experiments. Similarly to what observed in mice, caspase-4 was correlated to the stage of lung cancer in humans in that it induced cell proliferation in a K-Ras, c-MyC and IL-1α dependent manner. Caspase-4 positive adenocarcinoma (79.3%) and squamous carcinoma (88.2%) patients had lower median survival than patients who had lower levels of caspase-4. Moreover, PD-L1 expression and gene mutation (i.e. EGFR) were not correlated to caspase-4 expression. Instead, NSCLC patients who had K-Ras or c-MyC gene alteration were positively correlated to higher levels of caspase-4 and lower survival rate.
Conclusions: We identified a subgroup of NSCLC patients as caspase-4 positive among which double and triple positive caspase-4, K-Ras and/or c-MyC patients which prognosis was poor. Because K-Ras and c-MyC are still undrugable, the identification of caspase-4 as a novel oncoprotein could introduce novelty in the clinical yet unmet needs for NSCLC patients.
Keywords: Caspase-4; Cell proliferation; K-Ras; Lung cancer; Oncoprotein; Survival rate; Tumor progression; cMyc.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
MYC expression correlates with PD-L1 expression in non-small cell lung cancer.Lung Cancer. 2017 Aug;110:63-67. doi: 10.1016/j.lungcan.2017.06.006. Epub 2017 Jun 14. Lung Cancer. 2017. PMID: 28676221
-
Expression of TLR9 in tumor-infiltrating mononuclear cells enhances angiogenesis and is associated with a worse survival in lung cancer.Int J Cancer. 2014 Feb 15;134(4):765-77. doi: 10.1002/ijc.28413. Epub 2013 Sep 4. Int J Cancer. 2014. PMID: 23913633
-
Characterization of a genetic mouse model of lung cancer: a promise to identify Non-Small Cell Lung Cancer therapeutic targets and biomarkers.BMC Genomics. 2014;15 Suppl 3(Suppl 3):S1. doi: 10.1186/1471-2164-15-S3-S1. Epub 2014 May 6. BMC Genomics. 2014. PMID: 25077564 Free PMC article.
-
ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation.Theranostics. 2021 Jan 19;11(7):3392-3416. doi: 10.7150/thno.52435. eCollection 2021. Theranostics. 2021. PMID: 33537094 Free PMC article.
-
DDX5 promotes proliferation and tumorigenesis of non-small-cell lung cancer cells by activating β-catenin signaling pathway.Cancer Sci. 2015 Oct;106(10):1303-12. doi: 10.1111/cas.12755. Epub 2015 Sep 3. Cancer Sci. 2015. PMID: 26212035 Free PMC article.
Cited by
-
Pyroptosis: A Developing Foreland of Ovarian Cancer Treatment.Front Oncol. 2022 Feb 7;12:828303. doi: 10.3389/fonc.2022.828303. eCollection 2022. Front Oncol. 2022. PMID: 35198448 Free PMC article. Review.
-
Caspase-11 and AIM2 inflammasome are involved in smoking-induced COPD and lung adenocarcinoma.Oncotarget. 2021 May 25;12(11):1057-1071. doi: 10.18632/oncotarget.27964. eCollection 2021 May 25. Oncotarget. 2021. PMID: 34084280 Free PMC article.
-
Role of the AIM2 Inflammasome in Cancer: Potential Therapeutic Strategies.Biomedicines. 2025 Feb 6;13(2):395. doi: 10.3390/biomedicines13020395. Biomedicines. 2025. PMID: 40002808 Free PMC article. Review.
-
Deciphering a cell death-associated signature for predicting prognosis and response to immunotherapy in lung squamous cell carcinoma.Respir Res. 2023 Jul 6;24(1):176. doi: 10.1186/s12931-023-02402-9. Respir Res. 2023. PMID: 37415224 Free PMC article.
-
Pyroptosis-related genes regulate proliferation and invasion of pancreatic cancer and serve as the prognostic signature for modeling patient survival.Discov Oncol. 2022 May 28;13(1):39. doi: 10.1007/s12672-022-00495-0. Discov Oncol. 2022. PMID: 35633405 Free PMC article.
References
-
- Colarusso C, Terlizzi M, Molino A, Imitazione P, Somma P, Rega R, Saccomanno A, Aquino RP, Pinto A, Sorrentino R. AIM2 Inflammasome activation leads to IL-1α and TGF-β release from exacerbated chronic obstructive pulmonary disease-derived peripheral blood mononuclear cells. Front Pharmacol. 2019;10:257. doi: 10.3389/fphar.2019.00257. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

