JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality
- PMID: 33187978
- PMCID: PMC7775747
- DOI: 10.1126/sciadv.abe4724
JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality
Abstract
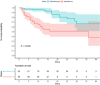
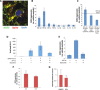
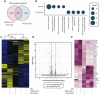
Using AI, we identified baricitinib as having antiviral and anticytokine efficacy. We now show a 71% (95% CI 0.15 to 0.58) mortality benefit in 83 patients with moderate-severe SARS-CoV-2 pneumonia with few drug-induced adverse events, including a large elderly cohort (median age, 81 years). An additional 48 cases with mild-moderate pneumonia recovered uneventfully. Using organotypic 3D cultures of primary human liver cells, we demonstrate that interferon-α2 increases ACE2 expression and SARS-CoV-2 infectivity in parenchymal cells by greater than fivefold. RNA-seq reveals gene response signatures associated with platelet activation, fully inhibited by baricitinib. Using viral load quantifications and superresolution microscopy, we found that baricitinib exerts activity rapidly through the inhibition of host proteins (numb-associated kinases), uniquely among antivirals. This reveals mechanistic actions of a Janus kinase-1/2 inhibitor targeting viral entry, replication, and the cytokine storm and is associated with beneficial outcomes including in severely ill elderly patients, data that incentivize further randomized controlled trials.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Paules C. I., Marston H. D., Fauci A. S., Coronavirus infections—More than just the common cold. JAMA 323, 707–708 (2020). - PubMed
-
- Wu Z., McGoogan J. M., Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323, 1239–1242 (2020). - PubMed
-
- Tanaka Y., Emoto K., Cai Z., Aoki T., Schlichting D., Rooney T., Macias W., Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis receiving background methotrexate therapy: A 12-week, double-blind, randomized placebo-controlled study. J. Rheumatol. 43, 504–511 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

