Cell-Type Specificity of Neuronal Excitability and Morphology in the Central Amygdala
- PMID: 33188006
- PMCID: PMC7877473
- DOI: 10.1523/ENEURO.0402-20.2020
Cell-Type Specificity of Neuronal Excitability and Morphology in the Central Amygdala
Abstract
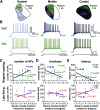
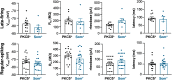
Central amygdala (CeA) neurons expressing protein kinase Cδ (PKCδ+) or somatostatin (Som+) differentially modulate diverse behaviors. The underlying features supporting cell-type-specific function in the CeA, however, remain unknown. Using whole-cell patch-clamp electrophysiology in acute mouse brain slices and biocytin-based neuronal reconstructions, we demonstrate that neuronal morphology and relative excitability are two distinguishing features between Som+ and PKCδ+ neurons in the laterocapsular subdivision of the CeA (CeLC). Som+ neurons, for example, are more excitable, compact, and with more complex dendritic arborizations than PKCδ+ neurons. Cell size, intrinsic membrane properties, and anatomic localization were further shown to correlate with cell-type-specific differences in excitability. Lastly, in the context of neuropathic pain, we show a shift in the excitability equilibrium between PKCδ+ and Som+ neurons, suggesting that imbalances in the relative output of these cells underlie maladaptive changes in behaviors. Together, our results identify fundamentally important distinguishing features of PKCδ+ and Som+ cells that support cell-type-specific function in the CeA.
Keywords: central amygdala; intrinsic excitability; morphology; neuropathic pain; protein kinase Cδ; somatostatin.
Copyright © 2021 Adke et al.
Figures










References
-
- Aggleton JP (2000) The amygdala: a functional analysis. New York: Oxford University Press.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
