Duchenne muscular dystrophy (DMD) cardiomyocyte-secreted exosomes promote the pathogenesis of DMD-associated cardiomyopathy
- PMID: 33188007
- PMCID: PMC7673361
- DOI: 10.1242/dmm.045559
Duchenne muscular dystrophy (DMD) cardiomyocyte-secreted exosomes promote the pathogenesis of DMD-associated cardiomyopathy
Abstract
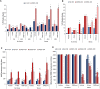
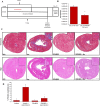
Cardiomyopathy is a leading cause of early mortality in Duchenne muscular dystrophy (DMD). There is a need to gain a better understanding of the molecular pathogenesis for the development effective therapies. Exosomes (exo) are secreted vesicles and exert effects via their RNA, lipid and protein cargo. The role of exosomes in disease pathology is unknown. Exosomes derived from stem cells have demonstrated cardioprotection in the murine DMD heart. However, it is unknown how the disease status of the donor cell type influences exosome function. Here, we sought to determine the phenotypic responses of DMD cardiomyocytes (DMD-iCMs) after long-term exposure to DMD cardiac exosomes (DMD-exo). DMD-iCMs were vulnerable to stress, evidenced by production of reactive oxygen species, the mitochondrial membrane potential and cell death levels. Long-term exposure to non-affected exosomes (N-exo) was protective. By contrast, long-term exposure to DMD-exo was not protective, and the response to stress improved with inhibition of DMD-exo secretion in vitro and in vivo The microRNA (miR) cargo, but not exosome surface peptides, was implicated in the pathological effects of DMD-exo. Exosomal surface profiling revealed N-exo peptides associated with PI3K-Akt signaling. Transcriptomic profiling identified unique changes with exposure to either N- or DMD-exo. Furthermore, DMD-exo miR cargo regulated injurious pathways, including p53 and TGF-beta. The findings reveal changes in exosomal cargo between healthy and diseased states, resulting in adverse outcomes. Here, DMD-exo contained miR changes, which promoted the vulnerability of DMD-iCMs to stress. Identification of these molecular changes in exosome cargo and effectual phenotypes might shed new light on processes underlying DMD cardiomyopathy.This article has an associated First Person interview with the first author of the paper.
Keywords: Cardiomyocyte; Cardiomyopathy; Exosome; MicroRNA; Stress; Vesicle.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsM.W.L. receives research support and is or recently has been a member of scientific advisory boards for Audentes Therapeutics, Solid Biosciences and Ichorion Therapeutics. M.W.L. is also a consultant for Audentes Therapeutics, Encoded Therapeutics, Modis Therapeutics, Lacerta Therapeutics, Biomarin, Prothelia, Affinia Therapeutics, Dynacure and AGADA Biosciences. All other authors have declared no competing interests.
Figures




References
-
- Afzal M. Z., Reiter M., Gastonguay C., Mcgivern J. V., Guan X., Ge Z.-D., Mack D. L., Childers M. K., Ebert A. D. and Strande J. L. (2016). Nicorandil, a nitric oxide donor and ATP-sensitive potassium channel opener, protects against dystrophin-deficient cardiomyopathy. J. Cardiovasc. Pharmacol. Ther. 21, 549 10.1177/1074248416636477 - DOI - PMC - PubMed
-
- Aminzadeh M. A., Rogers R. G., Fournier M., Tobin R. E., Guan X., Childers M. K., Andres A. M., Taylor D. J., Ibrahim A., Ding X. et al. (2018). Exosome-mediated benefits of cell therapy in mouse and human models of duchenne muscular dystrophy. Stem Cell Rep. 10, 942-955. 10.1016/j.stemcr.2018.01.023 - DOI - PMC - PubMed
-
- Bang C., Batkai S., Dangwal S., Gupta S. K., Foinquinos A., Holzmann A., Just A., Remke J., Zimmer K., Zeug A. et al. (2014). Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Invest. 124, 2136-2146. 10.1172/JCI70577 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

