Nanobubble-controlled nanofluidic transport
- PMID: 33188030
- PMCID: PMC7673748
- DOI: 10.1126/sciadv.abd0126
Nanobubble-controlled nanofluidic transport
Abstract
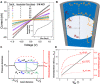
Nanofluidic platforms offering tunable material transport are applicable in biosensing, chemical detection, and filtration. Prior studies have achieved selective and controllable ion transport through electrical, optical, or chemical gating of complex nanostructures. Here, we mechanically control nanofluidic transport using nanobubbles. When plugging nanochannels, nanobubbles rectify and occasionally enhance ionic currents in a geometry-dependent manner. These conductance effects arise from nanobubbles inducing surface-governed ion transport through interfacial electrolyte films residing between nanobubble surfaces and nanopipette walls. The nanobubbles investigated here are mechanically generated, made metastable by surface pinning, and verified with cryogenic transmission electron microscopy. Our findings are relevant to nanofluidic device engineering, three-phase interface properties, and nanopipette-based applications.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Lohse D., Zhang X., Surface nanobubbles and nanodroplets. Rev. Mod. Phys. 87, 981–1035 (2015).
-
- Epstein P. S., Plesset M. S., On the stability of gas bubbles in liquid-gas solutions. J. Chem. Phys. 18, 1505–1509 (1950).
-
- Ljunggren S., Eriksson J. C., The lifetime of a colloid-sized gas bubble in water and the cause of the hydrophobic attraction. Colloid. Surface. A. 129–130, 151–155 (1997).
-
- Blander M., Katz J. L., Bubble nucleation in liquids. AIChE J. 21, 833–848 (1975).
-
- Zhang X., Chan D. Y. C., Wang D., Maeda N., Stability of interfacial nanobubbles. Langmuir 29, 1017–1023 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

