Combination of gemcitabine and anti-PD-1 antibody enhances the anticancer effect of M1 macrophages and the Th1 response in a murine model of pancreatic cancer liver metastasis
- PMID: 33188035
- PMCID: PMC7668383
- DOI: 10.1136/jitc-2020-001367
Combination of gemcitabine and anti-PD-1 antibody enhances the anticancer effect of M1 macrophages and the Th1 response in a murine model of pancreatic cancer liver metastasis
Abstract
Background: Pancreatic ductular adenocarcinoma (PDAC) is among the most dreadful of malignancies, in part due to the lack of efficacious chemotherapy. Immune checkpoint inhibitors, including anti-programmed cell death 1 (anti-PD-1) antibodies, are novel promising forms of systemic immunotherapy. In the current study, we assessed whether gemcitabine (GEM) combined with anti-PD-1 antibody treatment was efficacious as immunochemotherapy for advanced PDAC using a murine model of liver metastasis.
Methods: The murine model of PDAC liver metastasis was established by intrasplenically injecting the murine pancreatic cancer cell line PAN02 into immunocompetent C57BL/6J mice. The mice were treated with an anti-PD-1 antibody, GEM, or a combination of GEM plus anti-PD-1 antibody, and compared with no treatment (control); liver metastases, immune cell infiltration, gene expression, immune cell response phenotypes, and overall survival were investigated.
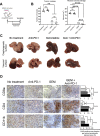
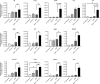
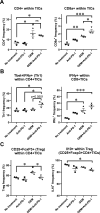
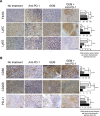
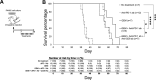
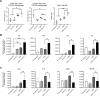
Results: In the metastatic tumor tissues of mice treated with GEM plus anti-PD-1 antibody, we observed the increased infiltration of Th1 lymphocytes and M1 macrophages. Gene expression profile analysis of peripheral blood cells obtained from mice treated with GEM plus anti-PD-1 antibody clearly highlighted T cell and innate immune signaling pathways. Survival of PDAC liver metastasis mice was significantly prolonged by the combination therapy (median survival, 66 days) when compared with that of GEM alone treatment (median survival, 56 days). Expanded lymphocytes, which were isolated from the splenocytes of PDAC liver metastasis mice treated with GEM plus anti-PD-1 antibody, had an increased number of M1 macrophages.
Conclusion: The combination of anti-PD-1 antibody immunotherapy with GEM was beneficial to treat a murine model of PDAC liver metastasis by enhancing the immune response mediated by Th1 lymphocytes and M1 macrophages and was associated with CD8+ T cells.
Keywords: combination; drug therapy; immunity; immunotherapy; macrophages; tumor microenvironment.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
