Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity
- PMID: 33188036
- PMCID: PMC7668384
- DOI: 10.1136/jitc-2020-001369
Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity
Abstract
Background: Immunotherapy represents the future of clinical cancer treatment. The type of cancer cell death determines the antitumor immune response and thereby contributes to the efficacy of anticancer therapy and long-term survival of patients. Induction of immunogenic apoptosis or necroptosis in cancer cells does activate antitumor immunity, but resistance to these cell death modalities is common. Therefore, it is of great importance to find other ways to kill tumor cells. Recently, ferroptosis has been identified as a novel, iron-dependent form of regulated cell death but whether ferroptotic cancer cells are immunogenic is unknown.
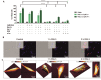
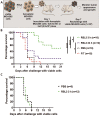
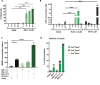
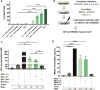
Methods: Ferroptotic cell death in murine fibrosarcoma MCA205 or glioma GL261 cells was induced by RAS-selective lethal 3 and ferroptosis was analyzed by flow cytometry, atomic force and confocal microscopy. ATP and high-mobility group box 1 (HMGB1) release were detected by luminescence and ELISA assays, respectively. Immunogenicity in vitro was analyzed by coculturing of ferroptotic cancer cells with bone-marrow derived dendritic cells (BMDCs) and rate of phagocytosis and activation/maturation of BMDCs (CD11c+CD86+, CD11c+CD40+, CD11c+MHCII+, IL-6, RNAseq analysis). The tumor prophylactic vaccination model in immune-competent and immune compromised (Rag-2-/-) mice was used to analyze ferroptosis immunogenicity.
Results: Ferroptosis can be induced in cancer cells by inhibition of glutathione peroxidase 4, as evidenced by confocal and atomic force microscopy and inhibitors' analysis. We demonstrate for the first time that ferroptosis is immunogenic in vitro and in vivo. Early, but not late, ferroptotic cells promote the phenotypic maturation of BMDCs and elicit a vaccination-like effect in immune-competent mice but not in Rag-2-/- mice, suggesting that the mechanism of immunogenicity is very tightly regulated by the adaptive immune system and is time dependent. Also, ATP and HMGB1, the best-characterized damage-associated molecular patterns involved in immunogenic cell death, have proven to be passively released along the timeline of ferroptosis and act as immunogenic signal associated with the immunogenicity of early ferroptotic cancer cells.
Conclusions: These results pave the way for the development of new therapeutic strategies for cancers based on induction of ferroptosis, and thus broadens the current concept of immunogenic cell death and opens the door for the development of new strategies in cancer immunotherapy.
Keywords: alarmins; cytotoxicity; immunogenicity; immunologic; immunotherapy; phagocytosis; vaccine.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
