Mapping the Dynamic Recruitment of Spinal Neurons during Fictive Locomotion
- PMID: 33188068
- PMCID: PMC7726538
- DOI: 10.1523/JNEUROSCI.1885-20.2020
Mapping the Dynamic Recruitment of Spinal Neurons during Fictive Locomotion
Abstract
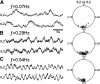
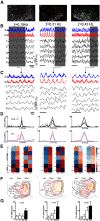
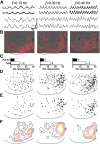
The basic rhythmic activity that underlies stepping is generated by a neural network, situated in the spinal cord, known as the locomotor central pattern generator (CPG). While a series of lesion experiments have demonstrated that the mammalian locomotor CPG is distributed throughout the ventral portion of the caudal spinal cord, the specific transverse distribution of this neural network is unclear. Here we evoke fictive locomotor activity of various frequencies in upright spinal cords prepared from male and female neonatal mice. This preparation enables us to use an imaging approach to identify locomotor-related cells across the transverse plane of the spinal cord. Results indicate that there is a clear shift in the recruitment of cells toward the ventromedial, and away from the ventrolateral, spinal cord as the frequency of fictive locomotion increases. Surprisingly, the analysis of multiple frequencies of fictive locomotion in the same spinal cord indicates that few neurons are involved in locomotor outputs across multiple speeds. Collectively, these experiments allow us to map the transverse distribution of the locomotor CPG and highlight the pattern of dynamic recruitment that occurs within this neural circuit as the frequency is altered. Our findings are consistent with data indicating that there is a speed-dependent recruitment of interneuronal populations during locomotion and suggest that the locomotor CPG is not a static network, but rather the specific cells recruited vary extensively based on demand.SIGNIFICANCE STATEMENT In this article, we use an imaging approach to identify all those cells that are rhythmically active at the same frequency as fictive locomotion recorded from the ventral roots of the isolated spinal cord. These experiments allow us to map the distribution of locomotor-related cells across the transverse plane of the spinal cord and identify the recruitment pattern of these cells as the frequency of locomotor outputs is altered. Our results indicate that there are drastic changes in the specific neurons activated at different frequencies and provide support for the concept that the locomotor central pattern generator is a modular network with speed-dependent recruitment of interneuronal components.
Keywords: CPG; interneuron; locomotion.
Copyright © 2020 the authors.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
