Characterization of the pathoimmunology of necrotizing enterocolitis reveals novel therapeutic opportunities
- PMID: 33188181
- PMCID: PMC7666196
- DOI: 10.1038/s41467-020-19400-w
Characterization of the pathoimmunology of necrotizing enterocolitis reveals novel therapeutic opportunities
Abstract
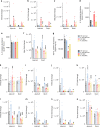
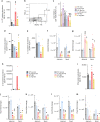
Necrotizing enterocolitis (NEC) is a severe, currently untreatable intestinal disease that predominantly affects preterm infants and is driven by poorly characterized inflammatory pathways. Here, human and murine NEC intestines exhibit an unexpected predominance of type 3/TH17 polarization. In murine NEC, pro-inflammatory type 3 NKp46-RORγt+Tbet+ innate lymphoid cells (ILC3) are 5-fold increased, whereas ILC1 and protective NKp46+RORγt+ ILC3 are obliterated. Both species exhibit dysregulation of intestinal TLR repertoires, with TLR4 and TLR8 increased, but TLR5-7 and TLR9-12 reduced. Transgenic IL-37 effectively protects mice from intestinal injury and mortality, whilst exogenous IL-37 is only modestly efficacious. Mechanistically, IL-37 favorably modulates immune homeostasis, TLR repertoires and microbial diversity. Moreover, IL-37 and its receptor IL-1R8 are reduced in human NEC epithelia, and IL-37 is lower in blood monocytes from infants with NEC and/or lower birthweight. Our results on NEC pathomechanisms thus implicate type 3 cytokines, TLRs and IL-37 as potential targets for novel NEC therapies.
Conflict of interest statement
Monash University, Hudson Institute (A.M.E., J.C.W., C.A.N.-P., and M.F.N.) and F. Hoffmann-La Roche (F.S., A.B., G.T. and L.L.) hold two patent families on IL-37, namely PCT/AU2016/050495 (Monash and Hudson only) and EP19218657.5 (Monash, Hudson, and Roche). No other conflicts of interest exist for these authors. All other authors declare that no conflict of interest exists.
Figures






References
-
- Blakely ML, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann. Surg. 2005;241:984–989. doi: 10.1097/01.sla.0000164181.67862.7f. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

