Vascular surveillance by haptotactic blood platelets in inflammation and infection
- PMID: 33188196
- PMCID: PMC7666582
- DOI: 10.1038/s41467-020-19515-0
Vascular surveillance by haptotactic blood platelets in inflammation and infection
Erratum in
-
Author Correction: Vascular surveillance by haptotactic blood platelets in inflammation and infection.Nat Commun. 2022 Aug 8;13(1):4645. doi: 10.1038/s41467-022-31310-7. Nat Commun. 2022. PMID: 35941111 Free PMC article. No abstract available.
Abstract
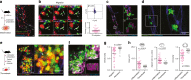
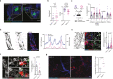
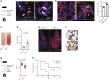
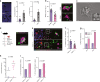
Breakdown of vascular barriers is a major complication of inflammatory diseases. Anucleate platelets form blood-clots during thrombosis, but also play a crucial role in inflammation. While spatio-temporal dynamics of clot formation are well characterized, the cell-biological mechanisms of platelet recruitment to inflammatory micro-environments remain incompletely understood. Here we identify Arp2/3-dependent lamellipodia formation as a prominent morphological feature of immune-responsive platelets. Platelets use lamellipodia to scan for fibrin(ogen) deposited on the inflamed vasculature and to directionally spread, to polarize and to govern haptotactic migration along gradients of the adhesive ligand. Platelet-specific abrogation of Arp2/3 interferes with haptotactic repositioning of platelets to microlesions, thus impairing vascular sealing and provoking inflammatory microbleeding. During infection, haptotaxis promotes capture of bacteria and prevents hematogenic dissemination, rendering platelets gate-keepers of the inflamed microvasculature. Consequently, these findings identify haptotaxis as a key effector function of immune-responsive platelets.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ho-Tin-Noé B, Boulaftali Y, Camerer E. Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood. 2018;131:277–288. - PubMed
-
- Semple JW, Italiano JE, Jr., Freedman J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011;11:264–274. - PubMed
-
- Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007;13:463–469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

