Multi-level analysis of reproduction in an Antarctic midge identifies female and male accessory gland products that are altered by larval stress and impact progeny viability
- PMID: 33188214
- PMCID: PMC7666147
- DOI: 10.1038/s41598-020-76139-6
Multi-level analysis of reproduction in an Antarctic midge identifies female and male accessory gland products that are altered by larval stress and impact progeny viability
Abstract
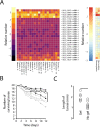
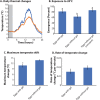
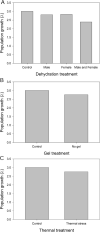
The Antarctic midge, Belgica antarctica, is a wingless, non-biting midge endemic to Antarctica. Larval development requires at least 2 years, but adults live only 2 weeks. The nonfeeding adults mate in swarms and females die shortly after oviposition. Eggs are suspended in a gel of unknown composition that is expressed from the female accessory gland. This project characterizes molecular mechanisms underlying reproduction in this midge by examining differential gene expression in whole males, females, and larvae, as well as in male and female accessory glands. Functional studies were used to assess the role of the gel encasing the eggs, as well as the impact of stress on reproductive biology. RNA-seq analyses revealed sex- and development-specific gene sets along with those associated with the accessory glands. Proteomic analyses were used to define the composition of the egg-containing gel, which is generated during multiple developmental stages and derived from both the accessory gland and other female organs. Functional studies indicate the gel provides a larval food source as well as a buffer for thermal and dehydration stress. All of these function are critical to juvenile survival. Larval dehydration stress directly reduces production of storage proteins and key accessory gland components, a feature that impacts adult reproductive success. Modeling reveals that bouts of dehydration may have a significant impact on population growth. This work lays a foundation for further examination of reproduction in midges and provides new information related to general reproduction in dipterans. A key aspect of this work is that reproduction and stress dynamics, currently understudied in polar organisms, are likely to prove critical in determining how climate change will alter their survivability.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Proteome profiling reveals tissue-specific protein expression in male and female accessory glands of the silkworm, Bombyx mori.Amino Acids. 2016 May;48(5):1173-83. doi: 10.1007/s00726-015-2141-8. Epub 2016 Jan 29. Amino Acids. 2016. PMID: 26822097
-
Genome sequencing of the winged midge, Parochlus steinenii, from the Antarctic Peninsula.Gigascience. 2017 Mar 1;6(3):1-8. doi: 10.1093/gigascience/giw009. Gigascience. 2017. PMID: 28327954 Free PMC article.
-
Rapid stress hardening in the Antarctic midge improves male fertility by increasing courtship success and preventing decline of accessory gland proteins following cold exposure.J Exp Biol. 2021 Jul 15;224(14):jeb242506. doi: 10.1242/jeb.242506. Epub 2021 Jul 23. J Exp Biol. 2021. PMID: 34297110
-
An integrative view of sexual selection in Tribolium flour beetles.Biol Rev Camb Philos Soc. 2008 May;83(2):151-71. doi: 10.1111/j.1469-185X.2008.00037.x. Biol Rev Camb Philos Soc. 2008. PMID: 18429767 Review.
-
Aquaporins in the antarctic midge, an extremophile that relies on dehydration for cold survival.Biol Bull. 2015 Aug;229(1):47-57. doi: 10.1086/BBLv229n1p47. Biol Bull. 2015. PMID: 26338869 Review.
Cited by
-
Exploring the molecular makeup of support cells in insect camera eyes.BMC Genomics. 2023 Nov 22;24(1):702. doi: 10.1186/s12864-023-09804-5. BMC Genomics. 2023. PMID: 37993800 Free PMC article.
-
Dehydration Dynamics in Terrestrial Arthropods: From Water Sensing to Trophic Interactions.Annu Rev Entomol. 2023 Jan 23;68:129-149. doi: 10.1146/annurev-ento-120120-091609. Epub 2022 Oct 21. Annu Rev Entomol. 2023. PMID: 36270273 Free PMC article. Review.
-
Reduced male fertility of an Antarctic mite following extreme heat stress could prompt localized population declines.Cell Stress Chaperones. 2023 Sep;28(5):541-549. doi: 10.1007/s12192-023-01359-4. Epub 2023 Jul 1. Cell Stress Chaperones. 2023. PMID: 37392307 Free PMC article.
-
External Morphology of Larvae of Belgica antarctica Jacobs, 1900 (Diptera, Chironomidae) Obtained from Two Locations in Maritime Antarctica.Insects. 2021 Sep 3;12(9):792. doi: 10.3390/insects12090792. Insects. 2021. PMID: 34564232 Free PMC article.
-
Obligate diapause and its termination shape the life-cycle seasonality of an Antarctic insect.Sci Rep. 2025 Feb 12;15(1):3890. doi: 10.1038/s41598-025-86617-4. Sci Rep. 2025. PMID: 39939619 Free PMC article.
References
-
- Convey P, Block W. Antarctic diptera: Ecology, physiology and distribution. Eur. J. Entomol. 1996;93:1–14.
-
- Sugg P, Edwards JS, Baust J. Phenology and life history of Belgica antarctica, an Antarctic midge (Diptera: Chironomidae) Ecol. Entomol. 1983;8:105–113. doi: 10.1111/j.1365-2311.1983.tb00487.x. - DOI
-
- Usher MB, Edwards M. A dipteran from south of the Antarctic circle: Belgica antarctica (Chironomidae) with a description of its larva. Biol. J. Lin. Soc. 1984;23:19–31. doi: 10.1111/j.1095-8312.1984.tb00803.x. - DOI
-
- Strong J. Ecology of terrestrial arthropods at Palmer Station, Antarctic Peninsula. In: Linsley-Gressitt J, editor. Entomology of Antarctica. Washington DC: American Geophysical Union; 1967. pp. 357–371.
-
- Edwards JS, Baust J. Sex ratio and adult behaviour of the Antarctic midge Belgica antarctica (Diptera, Chironomklae) Ecol. Entomol. 1981;6:239–243. doi: 10.1111/j.1365-2311.1981.tb00611.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

