COVID-19 and diabetes mellitus: from pathophysiology to clinical management
- PMID: 33188364
- PMCID: PMC7664589
- DOI: 10.1038/s41574-020-00435-4
COVID-19 and diabetes mellitus: from pathophysiology to clinical management
Abstract
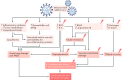
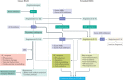
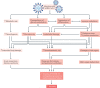
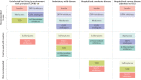
Initial studies found increased severity of coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in patients with diabetes mellitus. Furthermore, COVID-19 might also predispose infected individuals to hyperglycaemia. Interacting with other risk factors, hyperglycaemia might modulate immune and inflammatory responses, thus predisposing patients to severe COVID-19 and possible lethal outcomes. Angiotensin-converting enzyme 2 (ACE2), which is part of the renin-angiotensin-aldosterone system (RAAS), is the main entry receptor for SARS-CoV-2; although dipeptidyl peptidase 4 (DPP4) might also act as a binding target. Preliminary data, however, do not suggest a notable effect of glucose-lowering DPP4 inhibitors on SARS-CoV-2 susceptibility. Owing to their pharmacological characteristics, sodium-glucose cotransporter 2 (SGLT2) inhibitors might cause adverse effects in patients with COVID-19 and so cannot be recommended. Currently, insulin should be the main approach to the control of acute glycaemia. Most available evidence does not distinguish between the major types of diabetes mellitus and is related to type 2 diabetes mellitus owing to its high prevalence. However, some limited evidence is now available on type 1 diabetes mellitus and COVID-19. Most of these conclusions are preliminary, and further investigation of the optimal management in patients with diabetes mellitus is warranted.
Conflict of interest statement
S.L. has been a member of advisory boards or has consulted with Merck, Sharp & Dohme and NovoNordisk. S.L. has received grant support from AstraZeneca, Merck, Sharp & Dohme and Astellas. S.L. has also served on the speakers’ bureau of AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Merck, Sharp & Dohme, CKD Pharmaceutical and NovoNordisk. H.-S.K. has been a member of advisory boards or has consulted with Pfizer, Boehringer Ingelheim, Novartis, Daewoong Pharmaceutical, JW Pharmaceutical and NovoNordisk. H.-S.K. has received grant support from AstraZeneca. H.-S.K. has also served on the speakers’ bureau of Eli Lilly & Co., Merck, Sharp & Dohme, YUHAN, Dong-A Pharmaceutical and NovoNordisk. M.A.N. has been a member of advisory boards or has consulted with AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Fractyl, GlaxoSmithKline, Menarini/Berlin-Chemie, Merck, Sharp & Dohme and NovoNordisk. M.A.N. has received grant support from AstraZeneca, Eli Lilly & Co., Menarini/Berlin-Chemie, Merck, Sharp & Dohme, Novartis Pharma and NovoNordisk. M.A.N. has also served on the speakers’ bureau of AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Menarini/Berlin-Chemie, Merck, Sharp & Dohme, NovoNordisk and Sun Pharma. J.H.B. declares no competing interests.
Figures
Comment in
-
Autonomic dyshomeostasis in patients with diabetes mellitus during COVID-19.Nat Rev Endocrinol. 2021 Mar;17(3):189. doi: 10.1038/s41574-021-00466-5. Nat Rev Endocrinol. 2021. PMID: 33452491 Free PMC article. No abstract available.
-
Reply to: Autonomic dyshomeostasis in patients with diabetes mellitus during COVID-19.Nat Rev Endocrinol. 2021 Mar;17(3):189-190. doi: 10.1038/s41574-021-00467-4. Nat Rev Endocrinol. 2021. PMID: 33462400 Free PMC article. No abstract available.
References
-
- Faust JS, Del Rio C. Assessment of deaths from COVID-19 and from seasonal influenza. JAMA Intern. Med. 2020;180:1045–1046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

