Pattern of progression in advanced hepatocellular carcinoma treated with ramucirumab
- PMID: 33188713
- PMCID: PMC7898500
- DOI: 10.1111/liv.14731
Pattern of progression in advanced hepatocellular carcinoma treated with ramucirumab
Abstract
Background & aims: Radiological progression patterns to first-line sorafenib have been associated with post-progression and overall survival in advanced hepatocellular carcinoma, but these associations remain unknown for therapies in second- and later-line settings. This post hoc analysis of REACH and REACH-2 examined outcomes by radiological progression patterns in the second-line setting of patients with advanced hepatocellular carcinoma treated with ramucirumab or placebo.
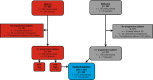
Methods: Patients with advanced hepatocellular carcinoma, Child-Pugh A and Eastern Cooperative Oncology Group Performance Status 0 or 1 with prior sorafenib were randomized to receive ramucirumab 8mg/kg or placebo every 2 weeks. Among 625 patients with ≥1 progression pattern (new extrahepatic lesion [including new macrovascular invasion], new intrahepatic lesion, extrahepatic growth or intrahepatic growth), data were analysed by trial and for pooled individual patient data for REACH-2 and REACH (alpha-fetoprotein ≥400 ng/mL). Cox models evaluated prognostic implications of progression patterns on overall and post-progression survival.
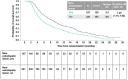
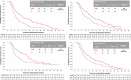
Results: Post-progression survival was worse among those with new extrahepatic lesions in REACH (HR 2.33, 95% CI 1.51-3.60), REACH-2 (HR 1.49, 95% CI 0.72-3.08) and the pooled population (HR 1.75, 95% CI 1.12-2.74) compared to other progression patterns. Overall survival was also significantly reduced in those with new extrahepatic lesions across studies. Ramucirumab provided an overall survival benefit across progression patterns, including patients with new extrahepatic lesions (HR 0.56, 95% CI 0.39-0.80) in the pooled population.
Conclusions: The emergence of new extrahepatic lesions in the second-line setting is a poor prognostic factor for post-progression survival. The benefit of ramucirumab for overall survival was consistent across progression patterns.
Trial registration: ClinicalTrials.gov NCT01140347 NCT02435433.
Keywords: best supportive care; disease progression patterns; new extrahepatic lesion; post-progression survival; ramucirumab.
© 2020 The Authors. Liver International published by John Wiley.
Conflict of interest statement
Maria Reig reports receiving grants and other from Bayer Schering Pharma and Ipsen; and receiving consulting fees from Bristol‐Meyers Squibb, Roche, Ipsen, AstraZeneca, Roche, Eli Lilly and Company and Gilead Sciences. Peter R. Galle reports receiving grants and personal fees from Bayer and personal fees from Bayer, Bristol‐Meyers Squibb, AstraZeneca, Sirtex, Merck Sharp & Dohme, Eisai, Ipsen and Roche, all outside of the submitted work. Masatoshi Kudo reports receiving personal fees and other from Bayer and Merck Sharp & Dohme; receiving grants, personal fees and other from Eisai Co., Ltd.; other from Ono Pharmaceutical; and receiving grants from Daiichi Sankyo, Medico's Hirata, Otsuka Pharmaceutical, Taiho Oncology, Astellas Pharma, Chugai Pharmaceutical, Bristol‐Myers Squibb, EA Pharma, Takeda and Gilead Sciences, all outside of the submitted work. Richard Finn reports serving as a consultant for AstraZeneca, Bayer, Bristol‐Myers Squibb, Eli Lilly and Company, Merck, Novartis and Roche/Genentech. Josep M. Llovet reports receiving research support from Bayer HealthCare Pharmaceuticals, Eisai Inc, Bristol‐Myers Squibb, Ipsen and Boehringer‐Ingelheim; and receiving consulting fees from Eisai Inc, Merck, Bayer HealthCare Pharmaceuticals, Bristol‐Myers Squibb, Celsion Corporation, Eli Lilly and Company, Ipsen, Glycotest, Roche, AstraZeneca, Sirtex, and Nucleix. Andrea L. Metti is an employee of Syneos Health who contracts with Eli Lilly and Company. William R. Schelman is a former employee and shareholder of Eli Lilly and Company. Kun Liang, Chunxiao Wang, Ryan C. Widau and Paolo Abada are current employees and shareholders of Eli Lilly and Company. Andrew X Zhu reports serving as a consultant/in an advisory role for Eisai Inc, Bristol‐Myers Squibb, Merck, Novartis, Sanofi, AstraZeneca, Bayer, Eli Lilly and Company and Exelixis; and receiving grants from Merck, Novartis, Bristol‐Myers Squibb, Bayer and Eli Lilly and Company.
Figures
References
-
- Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599‐616. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378‐390. - PubMed
-
- Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29(Suppl 4):iv238‐iv255. - PubMed
-
- United States Food and Drug Administration . FDA approves ramucirumab for hepatocellular carcinoma. https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐appro... [Accessed March 19, 2020]

