Traumatic brain injury modifies synaptic plasticity in newly-generated granule cells of the adult hippocampus
- PMID: 33188818
- PMCID: PMC7855349
- DOI: 10.1016/j.expneurol.2020.113527
Traumatic brain injury modifies synaptic plasticity in newly-generated granule cells of the adult hippocampus
Abstract
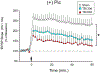
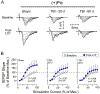
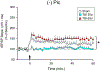
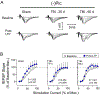
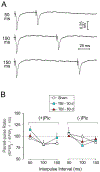
The hippocampus is vulnerable to traumatic brain injury (TBI), and hippocampal damage is associated with cognitive deficits that are often the hallmark of TBI. Recent studies have found that TBI induces enhanced neurogenesis in the dentate gyrus (DG) of the hippocampus, and this cellular response is related to innate cognitive recovery. However, cellular mechanisms of the role of DG neurogenesis in post-TBI recovery remain unclear. This study investigated changes in long-term potentiation (LTP) within the DG in relation to TBI-induced neurogenesis. Adult male rats received a moderate TBI or sham injury and were sacrificed for brain slice recordings at 30 or 60 days post-injury. Recordings were taken from the medial perforant path input to DG granule cells in the presence or absence of the GABAergic antagonist picrotoxin, reflecting activity of either all DG granule cells or predominately newborn granule cells, respectively. Measurements of LTP observed in the total granule cell population (with picrotoxin) showed a prolonged impairment which worsened between 30 and 60 days post-TBI. Under conditions which predominantly reflected the LTP elicited in newly born granule cells (no picrotoxin), a strikingly different pattern of post-TBI changes was observed, with a time-dependent cycle of functional impairment and recovery. At 30 days after injury this cell population showed little or no LTP, but by 60 days the capacity for LTP of the newly born granule cells was no different from that of sham controls. The time-frame of LTP improvements in the newborn cell population, comparable to that of behavioral recovery reported previously, suggests the unique functional properties of newborn granule cells enable them to contribute to restorative change following brain injury.
Keywords: Dentate gyrus; Long-term potentiation; Neurogenesis; Recovery of function; Traumatic brain injury.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus.J Neurophysiol. 2001 Jun;85(6):2423-31. doi: 10.1152/jn.2001.85.6.2423. J Neurophysiol. 2001. PMID: 11387388
-
Regional differences in GABAergic modulation for TEA-induced synaptic plasticity in rat hippocampal CA1, CA3 and dentate gyrus.Neurosci Res. 2007 Oct;59(2):183-90. doi: 10.1016/j.neures.2007.06.1472. Epub 2007 Jun 28. Neurosci Res. 2007. PMID: 17669533
-
Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery.Exp Neurol. 2007 Mar;204(1):264-72. doi: 10.1016/j.expneurol.2006.11.005. Epub 2007 Jan 2. Exp Neurol. 2007. PMID: 17198703
-
[Neuronal plasticity associated with learning and epileptic seizures: LTP and KIP].Seishin Shinkeigaku Zasshi. 2001;103(10):866-81. Seishin Shinkeigaku Zasshi. 2001. PMID: 11797444 Review. Japanese.
-
The effect of traumatic brain injury on learning and memory: A synaptic focus.Neuroscientist. 2025 Apr;31(2):195-214. doi: 10.1177/10738584241275583. Epub 2024 Sep 24. Neuroscientist. 2025. PMID: 39316552 Free PMC article. Review.
Cited by
-
Metformin attenuates sepsis-induced neuronal injury and cognitive impairment.BMC Neurosci. 2021 Dec 15;22(1):78. doi: 10.1186/s12868-021-00683-8. BMC Neurosci. 2021. PMID: 34911449 Free PMC article.
-
Acute and Chronic Neural and Glial Response to Mild Traumatic Brain Injury in the Hippocampus.bioRxiv [Preprint]. 2024 Apr 2:2024.04.01.587620. doi: 10.1101/2024.04.01.587620. bioRxiv. 2024. Update in: Biophys J. 2024 Oct 1;123(19):3346-3354. doi: 10.1016/j.bpj.2024.07.040. PMID: 38617329 Free PMC article. Updated. Preprint.
-
A single intranasal dose of human mesenchymal stem cell-derived extracellular vesicles after traumatic brain injury eases neurogenesis decline, synapse loss, and BDNF-ERK-CREB signaling.Front Mol Neurosci. 2023 May 22;16:1185883. doi: 10.3389/fnmol.2023.1185883. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37284464 Free PMC article.
-
Dendritic morphological development of traumatic brain injury-induced new neurons in the dentate gyrus is important for post-injury cognitive recovery and is regulated by Notch1.Exp Neurol. 2024 Dec;382:114963. doi: 10.1016/j.expneurol.2024.114963. Epub 2024 Sep 18. Exp Neurol. 2024. PMID: 39303845
-
Long-term potentiation and long-term depression are both impaired afterin vitrostretch injury measured with stretchable microelectrode arrays.Biomed Phys Eng Express. 2025 Jul 14;11(4):045029. doi: 10.1088/2057-1976/adea7e. Biomed Phys Eng Express. 2025. PMID: 40592347 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

