Weekly updates of national living evidence-based guidelines: methods for the Australian living guidelines for care of people with COVID-19
- PMID: 33188858
- PMCID: PMC7657075
- DOI: 10.1016/j.jclinepi.2020.11.005
Weekly updates of national living evidence-based guidelines: methods for the Australian living guidelines for care of people with COVID-19
Abstract
Background and objectives: The Australian National COVID-19 Clinical Evidence Taskforce is a consortium of 31 Australian health professional organisations developing living, evidence-based guidelines for care of people with COVID-19, which are updated weekly. This article describes the methods used to develop and maintain the guidelines.
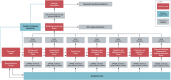
Methods: The guidelines use the GRADE methods and are designed to meet Australian NHMRC standards. Each week, new evidence is reviewed, current recommendations are revised, and new recommendations made. These are published in MAGIC and disseminated through traditional and social media. Relevant new questions to be addressed are continually sought from stakeholders and practitioners. For prioritized questions, the evidence is actively monitored and updated. Evidence surveillance combines horizon scans and targeted searches. An evidence team appraises and synthesizes evidence and prepares evidence-to-decision frameworks to inform development of recommendations. A guidelines leadership group oversees the development of recommendations by multidisciplinary guidelines panels and is advised by a consumer panel.
Results: The Taskforce formed in March 2020, and the first recommendations were published 2 weeks later. The guidelines have been revised and republished on a weekly basis for 24 weeks, and as of October 2020, contain over 90 treatment recommendations, suggesting that living methods are feasible in this context.
Conclusions: The Australian guidelines for care of people with COVID-19 provide an example of the feasibility of living guidelines and an opportunity to test and improve living evidence methods.
Keywords: COVID-19; Guidelines; Living evidence; Methods.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
COVID-19: living guidelines help fix cracks in evidence pipeline.Nature. 2021 Jul;595(7866):172. doi: 10.1038/d41586-021-01821-2. Nature. 2021. PMID: 34230655 No abstract available.
References
-
- Salyer K. COVID-19: what to know about the coronavirus pandemic on 6 April: World Economic Forum. 2020. https://www.weforum.org/agenda/2020/04/covid-19-what-to-know-about-the-c... Available at.
-
- Alderson L.J., Alderson P., Tan T. Median life span of a cohort of National Institute for Health and Care Excellence clinical guidelines was about 60 months. J Clin Epidemiol. 2014;67:52–55. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical