Photoreceptor cells and RPE contribute to the development of diabetic retinopathy
- PMID: 33188897
- PMCID: PMC8113320
- DOI: 10.1016/j.preteyeres.2020.100919
Photoreceptor cells and RPE contribute to the development of diabetic retinopathy
Abstract
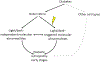
Diabetic retinopathy (DR) is a leading cause of blindness. It has long been regarded as vascular disease, but work in the past years has shown abnormalities also in the neural retina. Unfortunately, research on the vascular and neural abnormalities have remained largely separate, instead of being integrated into a comprehensive view of DR that includes both the neural and vascular components. Recent evidence suggests that the most predominant neural cell in the retina (photoreceptors) and the adjacent retinal pigment epithelium (RPE) play an important role in the development of vascular lesions characteristic of DR. This review summarizes evidence that the outer retina is altered in diabetes, and that photoreceptors and RPE contribute to retinal vascular alterations in the early stages of the retinopathy. The possible molecular mechanisms by which cells of the outer retina might contribute to retinal vascular damage in diabetes also are discussed. Diabetes-induced alterations in the outer retina represent a novel therapeutic target to inhibit DR.
Keywords: Diabetes; Diabetic retinopathy; Outer retina; Photoreceptors; Phototransduction; RPE; Vasculature; Visual cycle.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures






References
-
- Adams AJ, Zisman F, Ai E, Bresnick G, 1987. Macular Edema Reduces B Cone Sensitivity in Diabetics. Applied optics 26, 1455–1457. - PubMed
-
- Adler AJ, Southwick RE, 1992. Distribution of Glucose and Lactate in the Interphotoreceptor Matrix. Ophthalmic research 24, 243–252. - PubMed
-
- Agostinho P, Duarte CB, Oliveira CR, 1996. Activity of Ionotropic Glutamate Receptors in Retinal Cells: Effect of Ascorbate/Fe(2+)-Induced Oxidative Stress. J Neurochem 67, 1153–1163. - PubMed

