Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial
- PMID: 33189161
- PMCID: PMC7836724
- DOI: 10.1016/S2213-2600(20)30511-7
Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection carries a substantial risk of severe and prolonged illness; treatment options are currently limited. We assessed the efficacy and safety of inhaled nebulised interferon beta-1a (SNG001) for the treatment of patients admitted to hospital with COVID-19.
Methods: We did a randomised, double-blind, placebo-controlled, phase 2 pilot trial at nine UK sites. Adults aged 18 years or older and admitted to hospital with COVID-19 symptoms, with a positive RT-PCR or point-of-care test, or both, were randomly assigned (1:1) to receive SNG001 (6 MIU) or placebo by inhalation via a mouthpiece daily for 14 days. The primary outcome was the change in clinical condition on the WHO Ordinal Scale for Clinical Improvement (OSCI) during the dosing period in the intention-to-treat population (all randomised patients who received at least one dose of the study drug). The OSCI is a 9-point scale, where 0 corresponds to no infection and 8 corresponds to death. Multiple analyses were done to identify the most suitable statistical method for future clinical trials. Safety was assessed by monitoring adverse events for 28 days. This trial is registered with Clinicaltrialsregister.eu (2020-001023-14) and ClinicalTrials.gov (NCT04385095); the pilot trial of inpatients with COVID-19 is now completed.
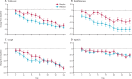
Findings: Between March 30 and May 30, 2020, 101 patients were randomly assigned to SNG001 (n=50) or placebo (n=51). 48 received SNG001 and 50 received placebo and were included in the intention-to-treat population. 66 (67%) patients required oxygen supplementation at baseline: 29 in the placebo group and 37 in the SNG001 group. Patients receiving SNG001 had greater odds of improvement on the OSCI scale (odds ratio 2·32 [95% CI 1·07-5·04]; p=0·033) on day 15 or 16 and were more likely than those receiving placebo to recover to an OSCI score of 1 (no limitation of activities) during treatment (hazard ratio 2·19 [95% CI 1·03-4·69]; p=0·043). SNG001 was well tolerated. The most frequently reported treatment-emergent adverse event was headache (seven [15%] patients in the SNG001 group and five [10%] in the placebo group). There were three deaths in the placebo group and none in the SNG001 group.
Interpretation: Patients who received SNG001 had greater odds of improvement and recovered more rapidly from SARS-CoV-2 infection than patients who received placebo, providing a strong rationale for further trials.
Funding: Synairgen Research.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures




Comment in
-
Nebulised interferon beta-1a for patients with COVID-19.Lancet Respir Med. 2021 Feb;9(2):122-123. doi: 10.1016/S2213-2600(20)30523-3. Epub 2020 Nov 12. Lancet Respir Med. 2021. PMID: 33189160 Free PMC article. No abstract available.
References
-
- Li S, Gong M, Zhao F, et al. Type I interferons: distinct biological activities and current applications for viral infection. Cell Physiol Biochem. 2018;51:2377–2396. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

