How to detect eosinophil ETosis (EETosis) and extracellular traps
- PMID: 33189567
- PMCID: PMC9333458
- DOI: 10.1016/j.alit.2020.10.002
How to detect eosinophil ETosis (EETosis) and extracellular traps
Abstract
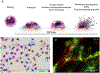
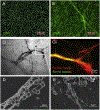
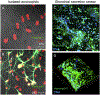
Eosinophils are short-lived and comprise only a small population of circulating leukocytes; however, they play surprisingly multifunctional roles in homeostasis and various diseases including allergy and infection. Recent research has shed light on active cytolytic eosinophil cell death that releases eosinophil extracellular traps (EETs) and total cellular contents, namely eosinophil extracellular trap cell death (EETosis). The pathological contribution of EETosis was made more cogent by recent findings that a classical pathological finding of eosinophilic inflammation, that of Charcot-Leyden crystals, is closely associated with EETosis. Currently no gold standard methods to identify EETosis exist, but "an active eosinophil lysis that releases cell-free granules and net-like chromatin structure" appears to be a common feature of EETosis. In this review, we describe several approaches that visualize EETs/EETosis in clinical samples and in vitro studies using isolated human eosinophils. EETs/EETosis can be observed using simple chemical or fluorescence staining, immunostaining, and electron microscopy, although it is noteworthy that visualization of EETs is greatly changed by sample preparation including the extracellular space of EETotic cells and shear flow. Considering the multiple aspects of biological significance, further study into EETs/EETosis is warranted to give a detailed understanding of the roles played in homeostasis and disease pathogenesis.
Keywords: Charcot-leyden crystal; EETosis; EETs; Eosinophil; NETs.
Copyright © 2020 Japanese Society of Allergology. Production and hosting by Elsevier B.V. All rights reserved.
Figures





References
-
- Nagase H, Ueki S, Fujieda S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol Int 2020;69:178–86. - PubMed
-
- Persson CG, Erjefalt JS. Eosinophil lysis and free granules: an in vivo paradigm for cell activation and drug development. Trends Pharmacol Sci 1997;18: 117–23. - PubMed
-
- Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, et al. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol 2018;141:571–85. e7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

