Ivermectin: An Anthelmintic, an Insecticide, and Much More
- PMID: 33189582
- PMCID: PMC7853155
- DOI: 10.1016/j.pt.2020.10.005
Ivermectin: An Anthelmintic, an Insecticide, and Much More
Abstract
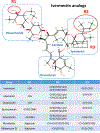
Here we tell the story of ivermectin, describing its anthelmintic and insecticidal actions and recent studies that have sought to reposition ivermectin for the treatment of other diseases that are not caused by helminth and insect parasites. The standard theory of its anthelmintic and insecticidal mode of action is that it is a selective positive allosteric modulator of glutamate-gated chloride channels found in nematodes and insects. At higher concentrations, ivermectin also acts as an allosteric modulator of ion channels found in host central nervous systems. In addition, in tissue culture, at concentrations higher than anthelmintic concentrations, ivermectin shows antiviral, antimalarial, antimetabolic, and anticancer effects. Caution is required before extrapolating from these preliminary repositioning experiments to clinical use, particularly for Covid-19 treatment, because of the high concentrations of ivermectin used in tissue-culture experiments.
Keywords: anthelmintic; anticancer; antimalaria; antivirus; insecticide; ivermectin.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures



References
-
- González Canga A et al. (2009) The pharmacokinetics and metabolism of ivermectin in domestic animal species. Vet. J. 179, 25–37 - PubMed
-
- Merck (2019) Stomectol safety data sheet. p. 7
-
- Campbell WC et al., eds (1989) Ivermectin and Abamectin, Springer-Verlag
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

