KALRN: A central regulator of synaptic function and synaptopathies
- PMID: 33189799
- PMCID: PMC7803032
- DOI: 10.1016/j.gene.2020.145306
KALRN: A central regulator of synaptic function and synaptopathies
Abstract
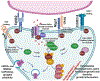
The synaptic regulator, kalirin, plays a key role in synaptic plasticity and formation of dendritic arbors and spines. Dysregulation of the KALRN gene has been linked to various neurological disorders, including autism spectrum disorder, Alzheimer's disease, schizophrenia, addiction and intellectual disabilities. Both genetic and molecular studies highlight the importance of normal KALRN expression for healthy neurodevelopment and function. This review aims to give an in-depth analysis of the structure and molecular mechanisms of kalirin function, particularly within the brain. These data are correlated to genetic evidence of patient mutations within KALRN and animal models of Kalrn that together give insight into the manner in which this gene may be involved in neurodevelopment and the etiology of disease. The emerging links to human disease from post-mortem, genome wide association (GWAS) and exome sequencing studies are examined to highlight the disease relevance of kalirin, particularly in neurodevelopmental diseases. Finally, we will discuss efforts to pharmacologically regulate kalirin protein activity and the implications of such endeavors for the treatment of human disease. As multiple disease states arise from deregulated synapse formation and altered KALRN expression and function, therapeutics may be developed to provide control over KALRN activity and thus synapse dysregulation. As such, a detailed understanding of how kalirin regulates neuronal development, and the manner in which kalirin dysfunction promotes neurological disease, may support KALRN as a valuable therapeutic avenue for future pharmacological intervention.
Keywords: Alzheimer’s disease; Autism spectrum disorder; Dendritic spine; Developmental delay; KALRN; Kalirin; Neurodegeneration; Neurodevelopment; Schizophrenia; Synaptic plasticity.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

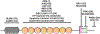

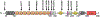
References
-
- Alam MR, et al. , 1997. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine alpha-amidating monooxygenase, an integral membrane peptide-processing enzyme. J. Biol. Chem 272 (19), 12667–12675. - PubMed
-
- Kawai T, Sanjo H, Akira S, 1999. Duet is a novel serine/threonine kinase with Dbl-Homology (DH) and Pleckstrin-Homology (PH) domains. Gene 227 (2), 249–255. - PubMed
-
- Colomer V, et al. , 1997. Huntingtin-associated protein 1 (HAP1) binds to a Trio-like polypeptide, with a rac1 guanine nucleotide exchange factor domain. Hum. Mol. Genet 6 (9), 1519–1525. - PubMed
-
- Alam MR, et al. , 1996. Novel proteins that interact with the COOH-terminal cytosolic routing determinants of an integral membrane peptide-processing enzyme. J. Biol. Chem 271 (45), 28636–28640. - PubMed
-
- Johnson RC, et al. , 2000. Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5’- and 3’-ends along with an internal translational initiation site. J. Biol. Chem 275 (25), 19324–19333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

