Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis
- PMID: 33189984
- PMCID: PMC7674615
- DOI: 10.1016/j.redox.2020.101771
Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis
Abstract
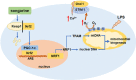
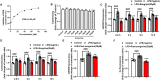
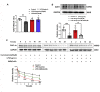
Septic cardiomyopathy is characterized by impaired contractive function with mitochondrial dysregulation. Songorine is a typical active C20-diterpene alkaloid from the lateral root of Aconitum carmichaelii, which has been used for the treatment of heart failure. This study investigated the protective role of songorine in septic heart injury from the aspect of mitochondrial biogenesis. Songorine (10, 50 mg/kg) protected cardiac contractive function against endotoxin insult in mice with Nrf2 induction. In cardiomyocytes, lipopolysaccharide (LPS) evoked mitochondrial reactive oxygen species (ROS) production and redistributed STIM1 to interact with Orai1 for the formation of calcium release-activated calcium (CRAC) channels, mediating calcium influx, which were prevented by songorine, likely due to ROS suppression. Songorine activated Nrf2 by promoting Keap1 degradation, having a contribution to enhancing antioxidant defenses. When LPS shifted metabolism away from mitochondrial oxidative phosphorylation (OXPHOS) in cardiomyocytes, songorine upregulated mitochondrial genes involved in fatty acid β-oxidation, tricarboxylic acid (TCA) cycle and electron transport chain in a manner dependent on Nrf2, resultantly protecting the capability of OXPHOS. Songorine increased luciferase report gene activities of nuclear respiratory factor-1 (Nrf1) and mitochondrial transcription factor A (Tfam) dependently on Nrf2, indicative of the regulation of Nrf2/ARE and NRF1 signaling cascades. Songorine promoted PGC-1α binding to Nrf2, and the cooperation was required for songorine to activate Nrf2/ARE and NRF1 for the control of mitochondrial quality and quantity. In support, the beneficial effects of songorine on cardioprotection and mitochondrial biogenesis were diminished by cardiac Nrf2 deficiency in mice subjected to LPS challenge. Taken together, these results showed that Nrf2 transcriptionally promoted mitochondrial biogenesis in cooperation with PGC-1α. Songorine activated Nrf2/ARE and NRF1 signaling cascades to rescue cardiomyocytes from endotoxin insult, suggesting that protection of mitochondrial biogenesis was a way for pharmacological intervention to prevent septic heart injury.
Keywords: Mitochondrial biogenesis; Nrf2; PGC-1α; Septic cardiomyopathy; Songorine.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures










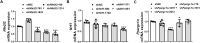


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

