Mesenchymal stem/stromal cells: Developmental origin, tumorigenesis and translational cancer therapeutics
- PMID: 33190044
- PMCID: PMC7672320
- DOI: 10.1016/j.tranon.2020.100948
Mesenchymal stem/stromal cells: Developmental origin, tumorigenesis and translational cancer therapeutics
Abstract
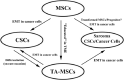
While a large and growing body of research has demonstrated that mesenchymal stem/stromal cells (MSCs) play a dual role in tumor growth and inhibition, studies exploring the capability of MSCs to contribute to tumorigenesis are rare. MSCs are key players during tumorigenesis and cancer development, evident in their faculty to increase cancer stem cells (CSCs) population, to generate the precursors of certain forms of cancer (e.g. sarcoma), and to induce epithelial-mesenchymal transition to create the CSC-like state. Indeed, the origin and localization of the native MSCs in their original tissues are not known. MSCs are identified in the primary tumor sites and the fetal and extraembryonic tissues. Acknowledging the developmental origin of MSCs and tissue-resident native MSCs is essential for better understanding of MSC contributions to the cellular origin of cancer. This review stresses that the plasticity of MSCs can therefore instigate further risk in select therapeutic strategies for some patients with certain forms of cancer. Towards this end, to explore the safe and effective MSC-based anti-cancer therapies requires a strong understanding of the cellular and molecular mechanisms of MSC action, ultimately guiding new strategies for delivering treatment. While clinical trial efforts using MSC products are currently underway, this review also provides new insights on the underlying mechanisms of MSCs to tumorigenesis and focuses on the approaches to develop MSC-based anti-cancer therapeutic applications.
Keywords: Cancer stem cell; Cancer/testis antigen; Epithelial-mesenchymal transition; Mesenchymal stem/stromal cell.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interests for this work.
Figures



References
-
- Molina E.R., Smith B.T., Shah S.R., Shin H., Mikos A.G. Immunomodulatory properties of stem cells and bioactive molecules for tissue engineering. J. Control. Release. 2015;219:107–118. - PubMed
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. - PubMed
-
- Viswanathan S., Shi Y., Galipeau J., Krampera M., Leblanc K., Martin I. Mesenchymal stem versus stromal cells: international Society for Cell & Gene Therapy (ISCT) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–1024. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

