Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial
- PMID: 33190147
- PMCID: PMC7667577
- DOI: 10.1001/jama.2020.22258
Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial
Abstract
Importance: It remains uncertain whether the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) reduce cardiovascular risk.
Objective: To determine the effects on cardiovascular outcomes of a carboxylic acid formulation of EPA and DHA (omega-3 CA) with documented favorable effects on lipid and inflammatory markers in patients with atherogenic dyslipidemia and high cardiovascular risk.
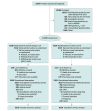
Design, setting, and participants: A double-blind, randomized, multicenter trial (enrollment October 30, 2014, to June 14, 2017; study termination January 8, 2020; last patient visit May 14, 2020) comparing omega-3 CA with corn oil in statin-treated participants with high cardiovascular risk, hypertriglyceridemia, and low levels of high-density lipoprotein cholesterol (HDL-C). A total of 13 078 patients were randomized at 675 academic and community hospitals in 22 countries in North America, Europe, South America, Asia, Australia, New Zealand, and South Africa.
Interventions: Participants were randomized to receive 4 g/d of omega-3 CA (n = 6539) or corn oil, which was intended to serve as an inert comparator (n = 6539), in addition to usual background therapies, including statins.
Main outcomes and measures: The primary efficacy measure was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina requiring hospitalization.
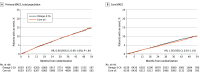
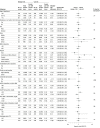
Results: When 1384 patients had experienced a primary end point event (of a planned 1600 events), the trial was prematurely halted based on an interim analysis that indicated a low probability of clinical benefit of omega-3 CA vs the corn oil comparator. Among the 13 078 treated patients (mean [SD] age, 62.5 [9.0] years; 35% women; 70% with diabetes; median low-density lipoprotein [LDL] cholesterol level, 75.0 mg/dL; median triglycerides level, 240 mg/dL; median HDL-C level, 36 mg/dL; and median high-sensitivity C-reactive protein level, 2.1 mg/L), 12 633 (96.6%) completed the trial with ascertainment of primary end point status. The primary end point occurred in 785 patients (12.0%) treated with omega-3 CA vs 795 (12.2%) treated with corn oil (hazard ratio, 0.99 [95% CI, 0.90-1.09]; P = .84). A greater rate of gastrointestinal adverse events was observed in the omega-3 CA group (24.7%) compared with corn oil-treated patients (14.7%).
Conclusions and relevance: Among statin-treated patients at high cardiovascular risk, the addition of omega-3 CA, compared with corn oil, to usual background therapies resulted in no significant difference in a composite outcome of major adverse cardiovascular events. These findings do not support use of this omega-3 fatty acid formulation to reduce major adverse cardiovascular events in high-risk patients.
Trial registration: ClinicalTrials.gov Identifier: NCT02104817.
Conflict of interest statement
Figures
Comment in
-
Effects of Omega-3 Fatty Acids on Major Adverse Cardiovascular Events: What Matters Most: the Drug, the Dose, or the Placebo?JAMA. 2020 Dec 8;324(22):2262-2264. doi: 10.1001/jama.2020.22387. JAMA. 2020. PMID: 33190148 No abstract available.
-
Do Omega-3 Fatty Acids Benefit Health?JAMA. 2020 Dec 8;324(22):2280-2281. doi: 10.1001/jama.2020.22898. JAMA. 2020. PMID: 33190151 No abstract available.
-
The REDUCE-IT verdict on eicosapentaenoic acid and cardiovascular outcome challenged with STRENGTH.Eur Heart J. 2021 Feb 1;42(5):370-371. doi: 10.1093/eurheartj/ehaa1042. Eur Heart J. 2021. PMID: 33367622 No abstract available.
-
In statin-treated patients at high CV risk, adding omega-3 fatty acids vs. corn oil to usual care did not reduce MACE.Ann Intern Med. 2021 Apr;174(4):JC40. doi: 10.7326/ACPJ202104200-040. Epub 2021 Apr 6. Ann Intern Med. 2021. PMID: 33819062
-
Omega-3 Fatty Acids Effect on Major Cardiovascular Events in Patients at High Cardiovascular Risk.JAMA. 2021 Apr 6;325(13):1333. doi: 10.1001/jama.2021.0830. JAMA. 2021. PMID: 33821903 No abstract available.
-
Omega-3 Fatty Acids Effect on Major Cardiovascular Events in Patients at High Cardiovascular Risk.JAMA. 2021 Apr 6;325(13):1334. doi: 10.1001/jama.2021.0827. JAMA. 2021. PMID: 33821904 No abstract available.
-
Omega-3 Fatty Acids Effect on Major Cardiovascular Events in Patients at High Cardiovascular Risk.JAMA. 2021 Apr 6;325(13):1332-1333. doi: 10.1001/jama.2021.0824. JAMA. 2021. PMID: 33821905 No abstract available.
-
Omega-3 Fatty Acids Effect on Major Cardiovascular Events in Patients at High Cardiovascular Risk.JAMA. 2021 Apr 6;325(13):1333. doi: 10.1001/jama.2021.0821. JAMA. 2021. PMID: 33821906 No abstract available.
References
-
- Del Gobbo LC, Imamura F, Aslibekyan S, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) . ω-3 Polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. 2016;176(8):1155-1166. doi: 10.1001/jamainternmed.2016.2925 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

