Gastrointestinal and renal complications in SARS-CoV-2-infected patients: Role of immune system
- PMID: 33190306
- PMCID: PMC7744842
- DOI: 10.1111/sji.12999
Gastrointestinal and renal complications in SARS-CoV-2-infected patients: Role of immune system
Abstract
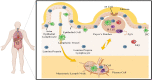
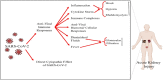
The recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease has been accompanied by various gastrointestinal (GI) and renal manifestations in significant portion of infected patients. Beside studies on the respiratory complications of coronavirus infection, understanding the essential immunological processes underlying the different clinical manifestations of virus infection is crucial for the identification and development of effective therapies. In addition to the respiratory tract, the digestive and urinary systems are the major sources of virus transmission. Thus, knowledge about the invasion mechanisms of SARS-CoV-2 in these systems and the immune system responses is important for implementing the infection prevention strategies. This article presents an overview of the gut and renal complications in SARS-CoV-2 infection. We focus on how SARS-CoV-2 interacts with the immune system and the consequent contribution of immune system, gut, and renal dysfunctions in the development of disease.
Keywords: SARS-CoV-2; antiviral immunity; gastrointestinal tract; kidney.
© 2020 The Scandinavian Foundation for Immunology.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

