Cancer Cell Resistance Against the Clinically Investigated Thiosemicarbazone COTI-2 Is Based on Formation of Intracellular Copper Complex Glutathione Adducts and ABCC1-Mediated Efflux
- PMID: 33190481
- PMCID: PMC7706001
- DOI: 10.1021/acs.jmedchem.0c01277
Cancer Cell Resistance Against the Clinically Investigated Thiosemicarbazone COTI-2 Is Based on Formation of Intracellular Copper Complex Glutathione Adducts and ABCC1-Mediated Efflux
Abstract
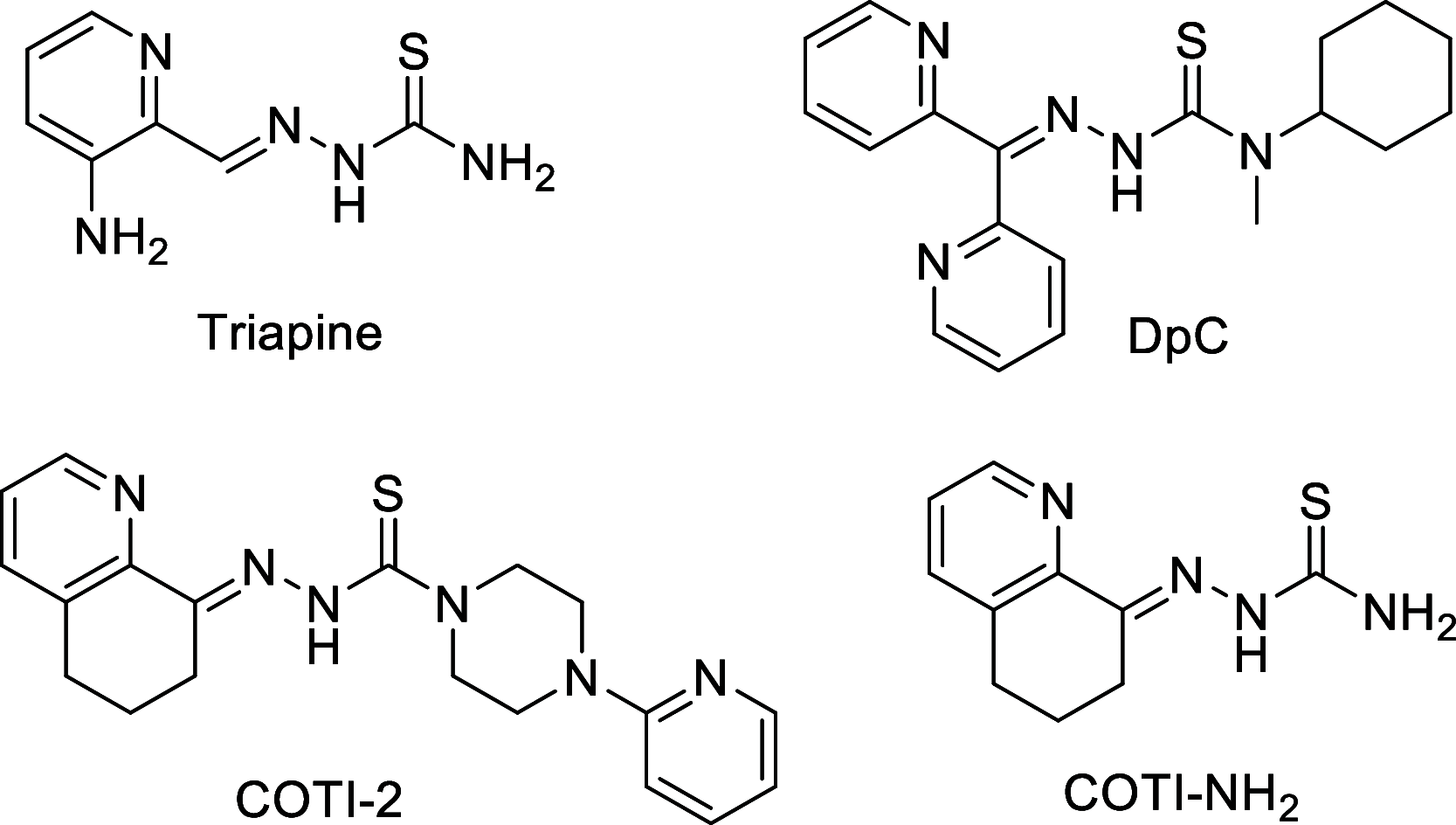
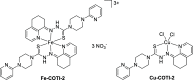
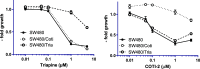
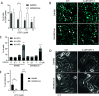
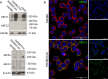
COTI-2 is a novel anticancer thiosemicarbazone in phase I clinical trial. However, the effects of metal complexation (a main characteristic of thiosemicarbazones) and acquired resistance mechanisms are widely unknown. Therefore, in this study, the copper and iron complexes of COTI-2 were synthesized and evaluated for their anticancer activity and impact on drug resistance in comparison to metal-free thiosemicarbazones. Investigations using Triapine-resistant SW480/Tria and newly established COTI-2-resistant SW480/Coti cells revealed distinct structure-activity relationships. SW480/Coti cells were found to overexpress ABCC1, and COTI-2 being a substrate for this efflux pump. This was unexpected, as ABCC1 has strong selectivity for glutathione adducts. The recognition by ABCC1 could be explained by the reduction kinetics of a ternary Cu-COTI-2 complex with glutathione. Thus, only thiosemicarbazones forming stable, nonreducible copper(II)-glutathione adducts are recognized and, in turn, effluxed by ABCC1. This reveals a crucial connection between copper complex chemistry, glutathione interaction, and the resistance profile of clinically relevant thiosemicarbazones.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
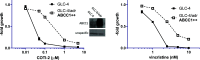


References
-
- Lobana T. S.; Sharma R.; Bawa G.; Khanna S. Bonding and structure trends of thiosemicarbazone derivatives of metals-An overview. Coord. Chem. Rev. 2009, 253, 977–1055. 10.1016/j.ccr.2008.07.004. - DOI
-
- Yu Y.; Kalinowski D. S.; Kovacevic Z.; Siafakas A. R.; Jansson P. J.; Stefani C.; Lovejoy D. B.; Sharpe P. C.; Bernhardt P. V.; Richardson D. R. Thiosemicarbazones from the old to new: Iron chelators that are more than just ribonucleotide reductase inhibitors. J. Med. Chem. 2009, 52, 5271–5294. 10.1021/jm900552r. - DOI - PubMed
-
- Lovejoy D. B.; Jansson P. J.; Brunk U. T.; Wong J.; Ponka P.; Richardson D. R. Antitumor Activity of Metal-Chelating Compound Dp44mT Is Mediated by Formation of a Redox-Active Copper Complex That Accumulates in Lysosomes. Cancer Res. 2011, 71, 5871–5880. 10.1158/0008-5472.CAN-11-1218. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

