Recent progress of antiviral therapy for coronavirus disease 2019
- PMID: 33190802
- PMCID: PMC7584884
- DOI: 10.1016/j.ejphar.2020.173646
Recent progress of antiviral therapy for coronavirus disease 2019
Abstract
The coronavirus disease 2019 (COVID-19) pandemic has become a global public health crisis, for which antiviral treatments are considered mainstream therapeutic approaches. With the development of this pandemic, the number of clinical studies on antiviral therapy, including remdesivir, chloroquine and hydroxychloroquine, lopinavir/ritonavir, ribavirin, arbidol, interferon, favipiravir, oseltamivir, nitazoxanide, nelfinavir, and camostat mesylate, has been increasing. However, the efficacy of these antiviral drugs for COVID-19 remains controversial. In this review, we summarize the recent progress and findings on antiviral therapies, aiming to provide clinical support for the management of COVID-19. In addition, we analyze the causes of controversy in antiviral drug research and discuss the quality of current studies on antiviral treatments. High-quality randomized clinical trials are required to demonstrate the efficacy and safety of antiviral drugs for the treatment of COVID-19.
Keywords: Antiviral therapy; COVID-19; SARS-CoV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures
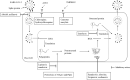
References
-
- Administration, T.U.S.F.a.D. Coronavirus (COVID-19) update. Daily Roundup May. 2020;1 2020.
-
- Authority M.A.H. 2020. The Seventh Edition of COVID-19 Diagnosis and Treatment Plan.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

