From thymus to periphery: Molecular basis of effector γδ-T cell differentiation
- PMID: 33191519
- PMCID: PMC7756812
- DOI: 10.1111/imr.12918
From thymus to periphery: Molecular basis of effector γδ-T cell differentiation
Abstract
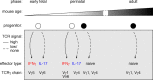
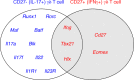
The contributions of γδ T cells to immune (patho)physiology in many pre-clinical mouse models have been associated with their rapid and abundant provision of two critical cytokines, interferon-γ (IFN-γ) and interleukin-17A (IL-17). These are typically produced by distinct effector γδ T cell subsets that can be segregated on the basis of surface expression levels of receptors such as CD27, CD44 or CD45RB, among others. Unlike conventional T cells that egress the thymus as naïve lymphocytes awaiting further differentiation upon activation, a large fraction of murine γδ T cells commits to either IFN-γ or IL-17 expression during thymic development. However, extrathymic signals can both regulate pre-programmed γδ T cells; and induce peripheral differentiation of naïve γδ T cells into effectors. Here we review the key cellular events of "developmental pre-programming" in the mouse thymus; and the molecular basis for effector function maintenance vs plasticity in the periphery. We highlight some of our contributions towards elucidating the role of T cell receptor, co-receptors (like CD27 and CD28) and cytokine signals (such as IL-1β and IL-23) in these processes, and the various levels of gene regulation involved, from the chromatin landscape to microRNA-based post-transcriptional control of γδ T cell functional plasticity.
Keywords: Gamma-delta T cells; IL-17; Thymic T cell development; effector T cell differentiation.
© 2020 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures


References
-
- Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336‐345. - PubMed
-
- Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836‐840. - PubMed
-
- Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T‐cell antigen receptors. Nature. 1988;335:443‐445. - PubMed
-
- Heyborne KD, Cranfill RL, Carding SR, Born WK, O'Brien RL. Characterization of gamma delta T lymphocytes at the maternal‐fetal interface. J Immunol. 1992;149:2872‐2878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

