NanoJ: a high-performance open-source super-resolution microscopy toolbox
- PMID: 33191949
- PMCID: PMC7655149
- DOI: 10.1088/1361-6463/ab0261
NanoJ: a high-performance open-source super-resolution microscopy toolbox
Abstract
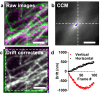
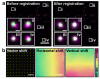
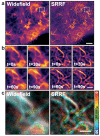
Super-resolution microscopy (SRM) has become essential for the study of nanoscale biological processes. This type of imaging often requires the use of specialised image analysis tools to process a large volume of recorded data and extract quantitative information. In recent years, our team has built an open-source image analysis framework for SRM designed to combine high performance and ease of use. We named it NanoJ-a reference to the popular ImageJ software it was developed for. In this paper, we highlight the current capabilities of NanoJ for several essential processing steps: spatio-temporal alignment of raw data (NanoJ-Core), super-resolution image reconstruction (NanoJ-SRRF), image quality assessment (NanoJ-SQUIRREL), structural modelling (NanoJ-VirusMapper) and control of the sample environment (NanoJ-Fluidics). We expect to expand NanoJ in the future through the development of new tools designed to improve quantitative data analysis and measure the reliability of fluorescent microscopy studies.
Keywords: Fiji; ImageJ; fluidics; image analysis; image quality assessment; single-particle analysis; super-resolution microscopy.
© 2019 IOP Publishing Ltd.
Figures




References
-
- Wheeler A, Henriques R. Standard and Super-Resolution Bioimaging Data Analysis: A Primer. New York: Wiley; 2017.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
